Electric Charges That Are Different Attract Each Other. True False
Juapaving
Mar 04, 2025 · 6 min read
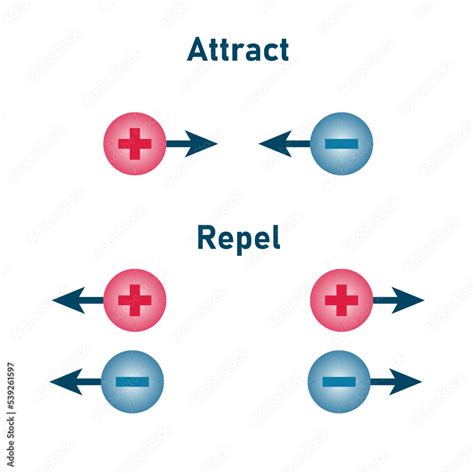
Table of Contents
Electric Charges: Opposites Attract – True or False? A Deep Dive into Electrostatics
The statement "electric charges that are different attract each other" is unequivocally true. This fundamental principle of electrostatics governs a vast array of phenomena, from the behavior of atoms to the operation of sophisticated electronic devices. Understanding this principle requires delving into the nature of electric charge, the forces between charges, and the implications of this attraction in the macroscopic world.
Understanding Electric Charge
At the heart of this principle lies the concept of electric charge, a fundamental property of matter. Objects possess electric charge due to an imbalance in the number of protons (positively charged) and electrons (negatively charged) within their constituent atoms. A neutral object has an equal number of protons and electrons, resulting in a net charge of zero. However, objects can become electrically charged through various processes like friction (triboelectric effect), conduction, or induction.
Types of Electric Charge: Positive and Negative
There are two fundamental types of electric charge: positive and negative. These charges are not arbitrary labels; they represent a fundamental dichotomy in the nature of electric interactions. Like charges repel each other, while unlike charges attract. This simple rule governs the intricate dance of charged particles at all scales, from subatomic particles to celestial bodies.
- Positive Charge: Typically associated with protons, a positive charge indicates an excess of protons compared to electrons.
- Negative Charge: Typically associated with electrons, a negative charge signifies an excess of electrons compared to protons.
Quantization of Charge
An important aspect of electric charge is its quantization. This means that electric charge exists in discrete units, multiples of the elementary charge, denoted as 'e'. The elementary charge is the magnitude of the charge of a single proton or electron, approximately 1.602 x 10⁻¹⁹ Coulombs. This means that an object cannot possess a fractional elementary charge; its total charge will always be an integer multiple of 'e'.
Coulomb's Law: Quantifying the Attraction
The strength of the attractive force between two oppositely charged objects is precisely described by Coulomb's Law. This law, a cornerstone of electrostatics, states that the force between two point charges is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
Mathematically, Coulomb's Law is expressed as:
F = k * |q₁q₂| / r²
Where:
- F represents the electrostatic force between the charges.
- k is Coulomb's constant (approximately 8.98755 × 10⁹ N⋅m²/C²).
- q₁ and q₂ are the magnitudes of the two charges.
- r is the distance between the centers of the two charges.
The absolute value signs around q₁q₂ indicate that the force is always attractive when the charges have opposite signs (one positive and one negative). The force is repulsive when the charges have the same sign (both positive or both negative).
Implications of Coulomb's Law
Coulomb's Law highlights several crucial aspects of electrostatic interactions:
- Strength of Attraction: The force of attraction is directly proportional to the magnitudes of the charges. Larger charges experience a stronger attractive force.
- Distance Dependence: The force weakens rapidly with increasing distance. Doubling the distance reduces the force to one-fourth its original value. This inverse square relationship is a hallmark of many fundamental forces in physics.
- Point Charges: The law strictly applies to point charges (charges concentrated at a single point). For extended objects, the calculation becomes more complex, often requiring integration techniques.
Examples of Opposite Charges Attracting
The attraction between oppositely charged objects is not a theoretical concept; it's a pervasive phenomenon observable in everyday life and crucial to many technological applications.
Static Electricity
The familiar "static cling" of clothes from a dryer or the shock you receive when touching a doorknob after walking across a carpet are prime examples of the attraction between oppositely charged objects. Friction between surfaces transfers electrons, creating regions of positive and negative charge that then attract each other.
Atoms and Molecules
The very structure of atoms and molecules is dictated by the electrostatic attraction between oppositely charged particles. The negatively charged electrons are attracted to the positively charged nucleus, holding the atom together. Similarly, the formation of chemical bonds often involves the electrostatic attraction between ions (charged atoms) or between polar molecules with partially positive and negative regions.
Capacitors
Capacitors are electronic components that store electrical energy by accumulating charge on two conductive plates separated by an insulator. The attraction between the oppositely charged plates allows for the storage of electrical energy. The capacitance, a measure of a capacitor's ability to store charge, is directly related to the area of the plates and the distance between them, reflecting Coulomb's law.
Batteries
Batteries operate on the principle of electrochemical reactions that generate a potential difference between two electrodes, creating a positive and negative terminal. The flow of electrons from the negative to the positive terminal generates an electric current. This process relies fundamentally on the electrostatic attraction between ions in the electrolyte and the electrodes.
Beyond Simple Attraction: A More Complex Picture
While the simple statement "electric charges that are different attract each other" holds true as a fundamental principle, the reality of electrostatic interactions is often more nuanced. Several factors can influence the behavior of charged objects:
Shielding Effects
The presence of other charged objects or conductive materials can significantly affect the electrostatic force between two charges. Conductive materials can effectively shield charges, reducing the net force experienced by a charge. This principle is exploited in Faraday cages, structures designed to protect sensitive equipment from external electric fields.
Polarization
Neutral objects can become polarized in the presence of an external electric field. This means that the distribution of charges within the object becomes distorted, with one side becoming slightly positive and the other slightly negative. This induced polarization can lead to attractive forces between a charged object and a neutral object. This effect is commonly observed with dielectric materials in capacitors.
Electric Fields
While Coulomb's Law describes the force directly, understanding electric fields provides a more comprehensive view of electrostatic interactions. An electric field is a region of space around a charged object where a force would be exerted on another charged object. The strength and direction of the electric field are determined by the distribution of charges. Opposite charges create electric fields that point toward each other, reinforcing the idea of attraction.
Conclusion: The Enduring Truth of Opposite Charges Attracting
The statement "electric charges that are different attract each other" remains fundamentally true and forms a cornerstone of our understanding of electrostatics. This simple principle underpins a vast array of phenomena, from the formation of atoms and molecules to the functioning of sophisticated electronic devices. While the complexities of shielding, polarization, and electric fields add layers of nuance, the basic principle of attraction between opposite charges persists as a powerful and essential concept in physics and engineering. Mastering this concept is crucial for comprehending the intricate world of electricity and its applications in our daily lives.
Latest Posts
Latest Posts
-
Number Of Valence Electrons In Magnesium
Mar 04, 2025
-
Which Is Greater Megabytes Or Gigabytes
Mar 04, 2025
-
What Is The Lcm For 10 And 12
Mar 04, 2025
-
Which Element Has Highest Ionization Energy
Mar 04, 2025
-
Least Common Factor Of 3 And 5
Mar 04, 2025
Related Post
Thank you for visiting our website which covers about Electric Charges That Are Different Attract Each Other. True False . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
