Number Of Valence Electrons In Magnesium
Juapaving
Mar 04, 2025 · 6 min read
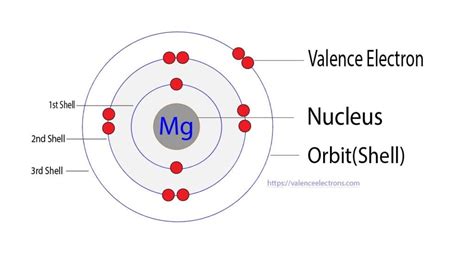
Table of Contents
Delving Deep into Magnesium: Unveiling its Valence Electrons and Chemical Behavior
Magnesium, a silvery-white alkaline earth metal, plays a crucial role in various biological and industrial processes. Understanding its electronic structure, particularly the number of valence electrons, is key to comprehending its reactivity and unique properties. This article delves deep into the world of magnesium's valence electrons, exploring its electronic configuration, chemical bonding, and the implications for its applications.
Understanding Valence Electrons: The Key to Reactivity
Before focusing specifically on magnesium, let's establish a foundational understanding of valence electrons. These are the electrons located in the outermost shell of an atom, also known as the valence shell. These electrons are the primary players in chemical reactions, determining an element's reactivity and the types of bonds it can form. The number of valence electrons dictates the atom's tendency to gain, lose, or share electrons to achieve a stable electron configuration, typically resembling a noble gas.
The Significance of the Octet Rule
The octet rule, a cornerstone of chemical bonding, states that atoms tend to gain, lose, or share electrons to achieve a full outer shell of eight electrons. This stable configuration, similar to that of noble gases, minimizes the atom's energy state. While there are exceptions, the octet rule provides a useful framework for understanding the chemical behavior of many elements, including magnesium.
Magnesium's Electronic Configuration: Unveiling the Valence Electrons
Magnesium (Mg), with an atomic number of 12, possesses 12 electrons. To determine the number of valence electrons, we need to examine its electronic configuration. Using the Aufbau principle and Hund's rule, we can systematically fill the electron orbitals:
- 1s² 2s² 2p⁶ 3s²
This configuration indicates that magnesium has two electrons in its first shell (1s²), eight electrons in its second shell (2s² 2p⁶), and two electrons in its third shell (3s²). The outermost shell, the third shell, contains two valence electrons. These two electrons are responsible for magnesium's chemical behavior and its ability to form chemical bonds.
Visualizing the Electron Configuration
Imagine the electron shells as concentric circles around the magnesium nucleus. The first shell holds a maximum of two electrons, the second shell eight, and the third shell can hold up to 18. In magnesium, the first two shells are completely filled, while the third shell contains only two electrons in the 3s orbital. These two lonely electrons in the outermost shell are eagerly seeking interaction to achieve a stable octet.
Magnesium's Chemical Bonding: A Consequence of its Valence Electrons
The presence of two valence electrons dictates magnesium's preferred method of bonding – it readily loses these two electrons to achieve a stable configuration, similar to neon (1s² 2s² 2p⁶). This tendency to lose electrons makes magnesium a highly reactive metal, readily participating in ionic bonding.
Ionic Bonding in Magnesium Compounds
Ionic bonding involves the electrostatic attraction between oppositely charged ions. When magnesium reacts with a non-metal, such as chlorine (Cl), it loses its two valence electrons to form a Mg²⁺ cation (positively charged ion). The chlorine atom gains one electron to form a Cl⁻ anion (negatively charged ion). The strong electrostatic attraction between the positively charged magnesium ion and the negatively charged chloride ions creates the ionic compound magnesium chloride (MgCl₂).
Examples of Magnesium's Ionic Bonding
Many common magnesium compounds demonstrate its tendency towards ionic bonding. These include:
- Magnesium oxide (MgO): Formed by the reaction of magnesium with oxygen. Magnesium loses two electrons to form Mg²⁺, while oxygen gains two electrons to form O²⁻.
- Magnesium hydroxide (Mg(OH)₂): A crucial component in antacids, formed by the reaction of magnesium with water.
- Magnesium sulfate (MgSO₄): Commonly used as Epsom salts, formed through the reaction of magnesium with sulfate ions.
Applications of Magnesium: Leveraging its Properties
The unique properties of magnesium, stemming directly from its electronic configuration and the resulting chemical behavior, lead to its wide-ranging applications across various industries:
1. Lightweight Alloys: Strength and Low Density
Magnesium's low density, approximately two-thirds the density of aluminum, makes it incredibly valuable in the aerospace and automotive industries. Magnesium alloys are used in aircraft components, automobile parts, and other applications where lightweight, high-strength materials are needed. The ability to form strong alloys is a direct result of the metallic bonding formed by magnesium's valence electrons.
2. Biomedical Applications: Biocompatibility and Degradation
Magnesium's biocompatibility and its ability to degrade within the body make it an ideal material for biodegradable implants, such as stents and bone screws. The controlled degradation process minimizes the need for secondary surgeries to remove implants.
3. Electronics Industry: Excellent Electrical Conductivity
Magnesium's excellent electrical conductivity finds application in various electronic components. It is used in batteries, as a sacrificial anode in cathodic protection systems, and in other applications requiring good electrical conductivity. This conductivity is a direct consequence of the relatively free movement of magnesium's valence electrons within the metallic lattice.
4. Metallurgy and Chemical Industry: Reducing Agent
The strong reducing power of magnesium, arising from its ease of losing electrons, makes it a valuable reducing agent in various metallurgical processes and chemical reactions. For instance, it’s used to extract other metals from their ores.
Beyond the Basics: Exploring Further Aspects of Magnesium's Chemistry
While we've focused primarily on magnesium's ionic bonding, it's worth noting that magnesium can also participate in other types of bonding under certain conditions. Though less common, magnesium can participate in some covalent bonding scenarios, forming bonds with elements with high electronegativity, slightly sharing electrons.
The Role of Oxidation States
Magnesium primarily exhibits a +2 oxidation state, reflecting the loss of its two valence electrons. This consistent oxidation state simplifies predictions of its chemical reactions and compound formation.
Magnesium's Reactivity and its Dependence on Valence Electrons
The reactivity of magnesium is directly linked to its two valence electrons. These electrons are easily lost, initiating chemical reactions and the formation of compounds. This reactivity is further influenced by factors like the presence of other elements and reaction conditions.
Conclusion: Understanding Magnesium Through its Valence Electrons
The number of valence electrons in magnesium, definitively two, dictates its chemical behavior, physical properties, and wide array of applications. By understanding its electronic configuration and how this impacts its bonding and reactivity, we gain a comprehensive appreciation for this essential element's unique role in numerous aspects of modern life, from the lightweight alloys in our vehicles to the biodegradable implants supporting our health. The seemingly simple number two – the number of magnesium's valence electrons – unlocks a world of complex chemical interactions and vital technological applications. Further research continues to reveal new and exciting applications of this versatile metal, driven by a deeper understanding of its fundamental chemical characteristics.
Latest Posts
Latest Posts
-
Which Of The Following Planets Has No Moon
Mar 04, 2025
-
The Swim Bladder Of Bony Fishes Functions In
Mar 04, 2025
-
What Is 3 5 As A Percentage
Mar 04, 2025
-
What Is The Difference Between Colonialism And Imperialism
Mar 04, 2025
-
Moment Of Inertia Of A Rod
Mar 04, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In Magnesium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
