Which Element Has Highest Ionization Energy
Juapaving
Mar 04, 2025 · 5 min read
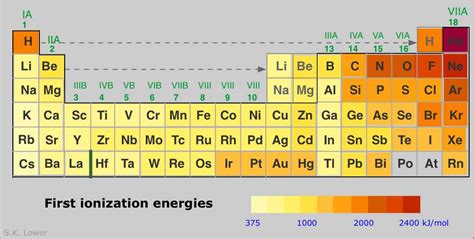
Table of Contents
Which Element Has the Highest Ionization Energy? A Deep Dive into Atomic Structure and Periodic Trends
The quest to identify the element boasting the highest ionization energy takes us on a fascinating journey through the intricacies of atomic structure and the periodic table. Understanding ionization energy itself is crucial; it's the energy required to remove an electron from a gaseous atom or ion. This seemingly simple process reveals much about the fundamental forces governing the behavior of matter. While a simple answer might seem readily available, delving into the nuances unveils a more complex and rewarding understanding of chemistry.
Understanding Ionization Energy
Ionization energy isn't a single value; rather, it's a series of values representing successive removals of electrons. The first ionization energy is the energy needed to remove the first electron from a neutral atom. The second ionization energy removes the second electron from the singly charged ion, and so on. Each subsequent ionization energy is always higher than the previous one. This is because removing an electron reduces the electron-electron repulsion within the atom, making it harder to remove subsequent electrons from the increasingly positive ion.
The magnitude of ionization energy depends heavily on several factors:
- Nuclear Charge: A greater number of protons in the nucleus (higher atomic number) leads to a stronger attraction for electrons, resulting in a higher ionization energy.
- Shielding Effect: Inner electrons partially shield outer electrons from the full nuclear charge. Electrons in lower energy levels effectively reduce the attraction felt by outer electrons, decreasing ionization energy.
- Atomic Radius: A smaller atomic radius means electrons are closer to the nucleus, experiencing a stronger attraction, and thus requiring more energy to remove. This is directly related to the shielding effect – less shielding means a smaller effective nuclear charge.
- Electron Configuration: The stability of electron configurations significantly impacts ionization energy. Atoms with full or half-filled subshells (like noble gases and some transition metals) exhibit higher ionization energies due to their enhanced stability.
Periodic Trends in Ionization Energy
The periodic table elegantly organizes elements based on their atomic structure and properties, reflecting clear trends in ionization energy:
-
Across a Period (Left to Right): Ionization energy generally increases as we move across a period from left to right. This is primarily due to the increasing nuclear charge. While the addition of electrons increases shielding, the effect of the increased nuclear charge is more dominant, resulting in stronger attraction to the electrons and thus higher ionization energy.
-
Down a Group (Top to Bottom): Ionization energy generally decreases as we move down a group. This is attributed to the increasing atomic radius. As we descend, electrons occupy higher energy levels, further away from the nucleus and shielded by increasing numbers of inner electrons. This reduced attraction makes it easier to remove an electron.
Helium: The Contender for Highest Ionization Energy
Considering the periodic trends, one might expect the element with the highest ionization energy to reside in the upper right corner of the periodic table. This points towards Helium (He). Helium possesses a small atomic radius, a high nuclear charge relative to its electron count (two protons and two electrons), and a stable, fully filled electron shell (1s²). All these factors contribute to an exceptionally high first ionization energy. It’s the most difficult element to ionize, requiring significant energy to remove its electrons.
While Helium's first ionization energy is significantly higher than other elements, the subsequent ionization energies follow the general pattern—the second ionization energy is substantially higher than the first. After the first electron is removed, the remaining electron experiences the full nuclear charge without any shielding, leading to a drastic increase in the energy required for ionization.
Exceptions and Nuances
While the general periodic trends provide a useful framework, there are exceptions and nuances:
-
Some anomalies exist within transition metals and post-transition metals. The irregular changes in ionization energies in these blocks are partly attributable to the involvement of d and f electrons in bonding, which shows less consistency in shielding effects than s and p electrons.
-
Full and Half-Filled Subshells: Elements with fully filled or half-filled subshells (like Nitrogen in the second period) display relatively higher ionization energies than their immediate neighbors because of their increased stability. Removing an electron from a stable configuration requires more energy.
-
Electron-Electron Repulsion: The repulsion between electrons also influences ionization energy. In some instances, increased electron-electron repulsion can slightly offset the increase in nuclear charge, leading to smaller-than-expected increases in ionization energy.
Beyond the First Ionization Energy
It is crucial to emphasize that focusing solely on the first ionization energy presents an incomplete picture. The successive ionization energies provide a far richer and detailed understanding of atomic structure. These values demonstrate how much more energy is progressively required to remove each successive electron, highlighting the increasing electrostatic attraction between the increasingly positive ion and remaining electrons.
Applications and Significance
The understanding of ionization energies is fundamental to many fields:
- Spectroscopy: Ionization energies are crucial in interpreting atomic spectra, as they correspond to specific energy transitions.
- Chemical Bonding: Ionization energies help explain the formation of chemical bonds, providing insights into the electron transfer or sharing processes between atoms.
- Materials Science: Understanding ionization energies is vital for designing and developing new materials with specific electronic properties.
- Astrophysics: Ionization energies are used to analyze the composition of stars and other celestial bodies.
Conclusion
While Helium takes the crown for having the highest first ionization energy, the story doesn't end there. The concept of ionization energy is far richer and more complex than a single value. Understanding the periodic trends, the influence of factors such as nuclear charge, shielding effect, and atomic radius, and acknowledging the exceptions to the general rules provides a much deeper appreciation for the fundamental principles governing atomic behavior and chemical reactivity. The study of ionization energies provides a window into the underlying structure of matter and its diverse properties, connecting seemingly simple atomic phenomena to a wide range of scientific disciplines. It underscores the importance of considering not only the first ionization energy but the entire sequence of successive ionization energies for a complete understanding of an element's behavior and its role in the universe.
Latest Posts
Latest Posts
-
What Is The Building Block Of All Matter
Mar 04, 2025
-
15 5 2b Equivalent Expression Worksheet
Mar 04, 2025
-
What Are Biotic Factors And Abiotic Factors
Mar 04, 2025
-
Which Of The Following Planets Has No Moon
Mar 04, 2025
-
The Swim Bladder Of Bony Fishes Functions In
Mar 04, 2025
Related Post
Thank you for visiting our website which covers about Which Element Has Highest Ionization Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
