Do Noble Gases Have High Ionization Energy
Juapaving
Mar 28, 2025 · 6 min read
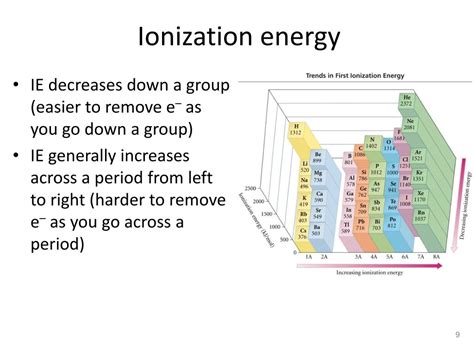
Table of Contents
Do Noble Gases Have High Ionization Energy? A Deep Dive into Atomic Structure and Reactivity
Noble gases, also known as inert gases, are a unique group of elements found in Group 18 of the periodic table. Their remarkable characteristic is their extreme chemical inertness, a property directly linked to their exceptionally high ionization energies. This article will delve into the reasons behind this high ionization energy, exploring the underlying atomic structure, electron configuration, and the implications of this property on the reactivity and applications of noble gases.
Understanding Ionization Energy
Ionization energy is the minimum amount of energy required to remove the most loosely bound electron from a neutral gaseous atom or ion. The first ionization energy refers to the removal of the first electron, the second ionization energy refers to the removal of the second electron, and so on. These energies are typically measured in kilojoules per mole (kJ/mol) or electronvolts (eV). The higher the ionization energy, the more difficult it is to remove an electron from the atom.
The Noble Gas Electron Configuration: The Key to Inertness
The key to understanding the high ionization energy of noble gases lies in their electron configuration. Noble gases possess a complete outermost electron shell, often referred to as a valence shell. This full valence shell, typically containing eight electrons (an octet, except for helium with two), is exceptionally stable. This stability arises from the quantum mechanical principles governing electron arrangement within an atom.
Specifically, the electrons in the outermost shell are subject to the least effective nuclear charge, meaning the positive charge of the nucleus is shielded by the inner electrons. In a noble gas, however, the full valence shell experiences a significantly stronger effective nuclear charge due to the balanced electron-proton interaction. This robust arrangement minimizes electron-electron repulsion and maximizes the overall stability of the atom. Removing an electron from this stable configuration requires a substantial amount of energy, resulting in their high ionization energies.
Helium: A Special Case
Helium, with only two electrons filling its 1s orbital, is a unique case. Although it doesn't follow the octet rule, its full electron shell contributes to its exceptional stability and high ionization energy. The 1s orbital is close to the nucleus, and the two electrons experience a strong attractive force from the nucleus. Therefore, removing an electron requires a significant amount of energy.
Comparing Ionization Energies Across the Periodic Table
The trend of ionization energy across the periodic table provides further insight into the unique position of noble gases. Ionization energy generally increases across a period (from left to right) as the effective nuclear charge increases, pulling the electrons more tightly towards the nucleus. It generally decreases down a group (from top to bottom) as the electrons are further from the nucleus and shielded by more inner electrons.
Noble gases stand out because they represent the peak of ionization energy within each period. The elements immediately preceding them have one electron less than a full valence shell, making it relatively easier to remove that electron. Similarly, the elements immediately following them in the next period begin with a new electron shell, and the outermost electron is further from the nucleus and more shielded, leading to lower ionization energy.
Factors Influencing Ionization Energy
Several factors contribute to the high ionization energy of noble gases beyond their electron configuration:
-
Effective Nuclear Charge: As mentioned earlier, the effective nuclear charge experienced by the valence electrons is significantly higher in noble gases due to the complete shell, making it harder to remove an electron.
-
Electron-Electron Repulsion: While a full valence shell minimizes electron-electron repulsion, removing an electron disrupts this balance and may actually lead to a slight decrease in repulsion. However, this effect is far outweighed by the strong attractive force from the nucleus.
-
Shielding Effect: Inner electrons shield the outer electrons from the full positive charge of the nucleus. However, in noble gases, this shielding effect is efficiently balanced by the strong nuclear pull on the full valence shell.
-
Orbital Penetration: The penetration of electrons into inner shells can also influence ionization energy. In noble gases, the electrons are tightly bound within their orbitals and are less likely to penetrate inner shells.
Implications of High Ionization Energy: Chemical Inertness
The exceptionally high ionization energy directly translates to the chemical inertness of noble gases. The high energy barrier required to remove an electron prevents them from readily participating in chemical reactions, which generally involve the transfer or sharing of electrons. This inertness is why they are often referred to as inert gases.
However, the term "inert" is not entirely accurate. While noble gases are remarkably unreactive under normal conditions, they can participate in chemical reactions under extreme conditions, such as high pressures and low temperatures, or through the use of powerful oxidizing agents. These reactions are generally less common and often involve the formation of compounds with highly electronegative elements like fluorine or oxygen.
Examples of Noble Gas Compounds: Challenging the Inert Label
Although rare, noble gas compounds do exist, demonstrating that even the most inert elements can participate in chemical bonding under specific circumstances. Some examples include Xenon hexafluoride (XeF6), Xenon tetrafluoride (XeF4), Krypton difluoride (KrF2), and Radon difluoride (RnF2). The formation of these compounds highlights the limitations of the simplistic notion of complete inertness and demonstrates the complex interplay of factors that govern chemical reactivity.
These compounds are generally formed with highly electronegative elements that can exert a strong polarizing effect on the noble gas atoms, making it easier to remove or share electrons. Furthermore, the use of extreme conditions such as high pressures or energy input provides the necessary activation energy to overcome the high ionization energy barrier.
Applications of Noble Gases: Leveraging their Unique Properties
The high ionization energy and consequent chemical inertness of noble gases are the basis for many of their important applications. Their stability makes them suitable for a variety of uses in various fields, including:
-
Lighting: Noble gases are widely used in lighting applications, such as neon signs and fluorescent lamps. The characteristic colors emitted when noble gases are excited by an electric current are used to create visually appealing and functional lighting.
-
Welding: Inert gases like Argon and Helium are used as shielding gases in welding processes. Their inertness prevents the reaction of the molten metal with the surrounding atmosphere, ensuring high-quality welds.
-
Cryogenics: Helium is used in cryogenics due to its extremely low boiling point. Its inertness ensures it does not react with the materials being cooled.
-
Medical applications: Helium and other noble gases find applications in medical imaging and treatments.
-
Scientific research: Noble gases are frequently utilized in scientific research, especially in fields such as spectroscopy and nuclear magnetic resonance (NMR).
Conclusion: High Ionization Energy as a Defining Characteristic
The high ionization energy of noble gases is a fundamental property stemming from their complete valence electron shells and strong effective nuclear charge. This property dictates their chemical inertness, a characteristic responsible for their extensive applications across various industries and scientific fields. While not entirely "inert" under extreme conditions, their resistance to chemical reactions and their unique physical properties make noble gases invaluable resources in modern science and technology. Understanding their atomic structure and the factors influencing their ionization energy provides a comprehensive appreciation of this remarkable group of elements.
Latest Posts
Latest Posts
-
Why Is Cellulose Not A Source Of Nutrients For Humans
Mar 30, 2025
-
Law Of Conservation Of Mass States That
Mar 30, 2025
-
Simplify The Square Root Of 12
Mar 30, 2025
-
Find The Area Under The Curve Over The Interval
Mar 30, 2025
-
What Percent Is Equal To 3 8
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Do Noble Gases Have High Ionization Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
