Law Of Conservation Of Mass States That
Juapaving
Mar 30, 2025 · 6 min read
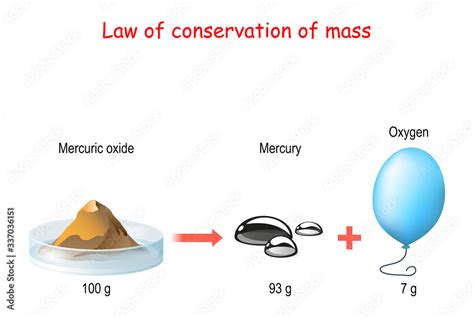
Table of Contents
The Law of Conservation of Mass: A Deep Dive into Matter's Unchanging Quantity
The Law of Conservation of Mass, a cornerstone of chemistry and physics, asserts that matter cannot be created or destroyed in a chemical reaction. While this statement seems straightforward, its implications are far-reaching and have shaped our understanding of the universe. This comprehensive exploration will delve into the intricacies of this fundamental law, exploring its historical context, scientific basis, applications, limitations, and its enduring relevance in modern science.
A Historical Perspective: From Alchemy to Modern Chemistry
The concept of conservation wasn't always so clearly defined. Early alchemists, striving to transmute base metals into gold, often observed changes in mass during their experiments. However, their rudimentary techniques and incomplete understanding of chemical reactions prevented them from accurately interpreting these changes. They lacked the precision instruments and theoretical framework necessary to formulate a universally applicable law.
The true foundation for the Law of Conservation of Mass was laid by Antoine Lavoisier, a prominent 18th-century French chemist, often hailed as the "father of modern chemistry." Through meticulous experimentation, Lavoisier demonstrated that in a closed system, the total mass of reactants is always equal to the total mass of products. He meticulously weighed substances before and after chemical reactions, consistently observing this equality. His experiments involved a wide range of chemical processes, solidifying the law's broad applicability. His work marked a significant shift from the qualitative observations of alchemists to the quantitative measurements that define modern science. Lavoisier's emphasis on precise measurement and careful experimentation established a rigorous scientific approach that is still central to scientific inquiry today.
The Scientific Basis: Understanding Atoms and Molecules
The Law of Conservation of Mass finds its firmest basis in the atomic theory of matter. This theory posits that matter is composed of indivisible particles called atoms. Chemical reactions, therefore, involve the rearrangement of atoms, not their creation or destruction. Atoms maintain their identity throughout a chemical transformation; they are neither gained nor lost.
Consider a simple reaction like the combustion of methane (CH₄) with oxygen (O₂):
CH₄ + 2O₂ → CO₂ + 2H₂O
In this reaction, one molecule of methane reacts with two molecules of oxygen to produce one molecule of carbon dioxide and two molecules of water. While the arrangement of atoms changes, the total number of each type of atom remains constant. There are one carbon atom, four hydrogen atoms, and four oxygen atoms on both sides of the equation. This illustrates the core principle of the Law of Conservation of Mass: the total mass of the reactants (methane and oxygen) equals the total mass of the products (carbon dioxide and water).
Applications: From Everyday Life to Complex Scientific Processes
The Law of Conservation of Mass underpins countless applications across various scientific disciplines and everyday life.
1. Stoichiometry: Quantifying Chemical Reactions
Stoichiometry, a crucial branch of chemistry, relies entirely on the Law of Conservation of Mass. It allows chemists to calculate the precise amounts of reactants needed to produce a desired amount of product, and vice-versa. This is essential in various industrial processes, pharmaceutical manufacturing, and environmental studies.
2. Balancing Chemical Equations: Ensuring Mass Conservation
Balancing chemical equations, a fundamental skill for any chemist, directly reflects the Law of Conservation of Mass. The process of ensuring an equal number of atoms of each element on both sides of the equation ensures that mass is conserved throughout the reaction.
3. Industrial Processes: Optimizing Efficiency and Yield
Industrial chemical processes are designed with the Law of Conservation of Mass in mind. Optimizing reactant ratios and minimizing waste products are crucial for maximizing efficiency and yield, all based on the principle of mass conservation.
4. Environmental Studies: Monitoring Pollutant Levels
Tracking the movement and transformation of pollutants in the environment requires understanding mass conservation. Tracing the fate of pollutants and predicting their environmental impact depends on accurate mass balances.
5. Forensic Science: Analyzing Crime Scenes
Forensic science often involves analyzing the mass of substances found at crime scenes. The principle of mass conservation helps investigators understand the processes involved in the crime and reconstruct events.
Limitations and Nuances: Where the Law Breaks Down
While the Law of Conservation of Mass is remarkably robust, it does have limitations.
1. Nuclear Reactions: Mass-Energy Equivalence
The law breaks down in nuclear reactions where a small amount of mass is converted into energy, as described by Einstein's famous equation, E=mc². In nuclear fission or fusion, the total mass of the products slightly differs from the total mass of the reactants. This mass difference is converted into a substantial amount of energy. This phenomenon necessitates the broader Law of Conservation of Mass-Energy.
2. Open Systems: Mass Exchange with Surroundings
The Law of Conservation of Mass strictly applies only to closed systems – systems that do not exchange matter with their surroundings. In open systems, matter can enter or leave, making it impossible to accurately track mass changes. For instance, a reaction occurring in an open container can lose volatile products, leading to apparent mass loss.
3. Extremely High Pressures and Temperatures: Relativistic Effects
At extremely high pressures and temperatures, such as those found in stellar interiors, relativistic effects can become significant. These effects can slightly alter the relationship between mass and energy, leading to deviations from the classical Law of Conservation of Mass.
The Enduring Relevance: Beyond Classical Chemistry
Despite its limitations in specific contexts, the Law of Conservation of Mass remains a fundamental concept in chemistry and physics. It forms the bedrock for understanding chemical reactions and serves as a powerful tool for quantitative analysis. Even with the broader Law of Conservation of Mass-Energy, the concept of mass conservation remains crucial for understanding many everyday phenomena and complex scientific processes.
The law's enduring relevance lies in its simplicity and broad applicability. Its straightforward principle provides a framework for interpreting countless natural phenomena and for designing and optimizing various industrial processes. Its role in stoichiometry and chemical equation balancing ensures that chemistry remains a predictive and quantifiable science.
Moreover, the historical development of the Law of Conservation of Mass highlights the importance of meticulous experimentation and the iterative process of refining scientific theories. Lavoisier’s rigorous approach and his emphasis on quantitative data established a scientific methodology that is still followed today. His work serves as a powerful example of how careful observation and rigorous experimentation can lead to groundbreaking discoveries and fundamental scientific laws.
Conclusion: A Fundamental Law for a Changing World
In conclusion, the Law of Conservation of Mass stands as a testament to the elegant simplicity and profound implications of fundamental scientific principles. While it has its limitations in extreme scenarios, its core principle remains a cornerstone of chemistry and physics. Its continued application across a vast spectrum of disciplines underlines its lasting significance in our understanding of the natural world. From balancing chemical equations to understanding complex environmental processes, the law’s enduring impact emphasizes its fundamental importance in both classical and modern science. The lessons learned from its historical development continue to inspire and guide scientific inquiry, highlighting the value of precision, meticulousness, and a constant pursuit of knowledge.
Latest Posts
Latest Posts
-
What Is The Function Of The Collecting Duct
Apr 01, 2025
-
A Rectangle Is A Square Always Sometimes Never
Apr 01, 2025
-
What Are The First 5 Multiples Of 2
Apr 01, 2025
-
What Is The Numeral For 42
Apr 01, 2025
-
Butter Melting Is A Physical Change
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Law Of Conservation Of Mass States That . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
