Differentiate Between Homogeneous And Heterogeneous Mixtures With Examples
Juapaving
Mar 24, 2025 · 6 min read
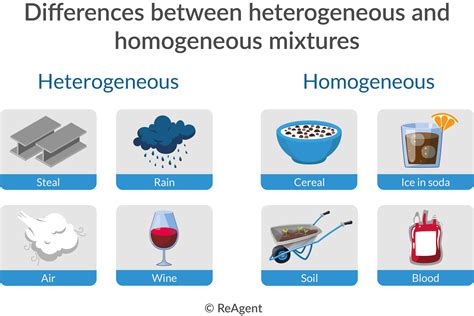
Table of Contents
Differentiating Homogeneous and Heterogeneous Mixtures: A Comprehensive Guide
Understanding the difference between homogeneous and heterogeneous mixtures is fundamental to various scientific disciplines, from chemistry and physics to materials science and environmental studies. This comprehensive guide will delve deep into the characteristics of each, providing clear definitions, illustrative examples, and practical applications. We'll also explore the methods used to separate components of these mixtures.
What is a Mixture?
Before diving into the specifics of homogeneous and heterogeneous mixtures, let's define what a mixture is. A mixture is a substance composed of two or more components not chemically bonded. A key characteristic is that the components retain their individual chemical properties. This contrasts with compounds, where components undergo chemical reactions to form new substances with different properties. Mixtures can be separated into their constituent parts through physical methods, unlike compounds which require chemical reactions.
Homogeneous Mixtures: Uniformity at the Macroscopic Level
A homogeneous mixture is a type of mixture where the composition is uniform throughout. This means that at a macroscopic level (meaning visible to the naked eye or with a standard microscope), the mixture appears to be one substance. The individual components are indistinguishable, even if you zoom in. The ratio of the components is constant regardless of the sample size.
Characteristics of Homogeneous Mixtures:
- Uniform composition: The components are evenly distributed throughout the mixture.
- Single phase: A homogeneous mixture exists in a single phase, whether solid, liquid, or gas. You won't see distinct layers or regions of different composition.
- Invisible components: The individual components cannot be visually distinguished from one another.
Examples of Homogeneous Mixtures:
- Air: A mixture of primarily nitrogen, oxygen, argon, and trace amounts of other gases. While each gas retains its properties, air appears as a single, uniform substance.
- Saltwater: When table salt (sodium chloride) dissolves completely in water, it forms a homogeneous mixture. The salt ions are evenly distributed throughout the water.
- Sugar water: Similar to saltwater, dissolving sugar in water creates a homogeneous solution where the sugar molecules are uniformly dispersed.
- Steel: An alloy of iron and carbon, steel is a homogeneous mixture where the carbon atoms are uniformly distributed within the iron matrix.
- Brass: This alloy of copper and zinc also represents a homogeneous mixture with a uniform distribution of the two metals.
- Vinegar: A solution of acetic acid in water is another example of a common homogeneous mixture.
Heterogeneous Mixtures: A Visible Variety of Components
In contrast to homogeneous mixtures, a heterogeneous mixture exhibits non-uniform composition. This means that the components are not evenly distributed throughout the mixture, and different regions have different compositions. The different components are easily visible to the naked eye or under a microscope.
Characteristics of Heterogeneous Mixtures:
- Non-uniform composition: The components are not evenly distributed, resulting in visibly different regions.
- Multiple phases: Heterogeneous mixtures often have multiple phases, meaning you can see distinct regions of different composition or physical states.
- Visible components: The individual components are easily discernible from each other.
Examples of Heterogeneous Mixtures:
- Sand and water: When sand is mixed with water, the sand particles settle at the bottom, creating distinct layers. This is a clear example of a heterogeneous mixture with two visibly different phases.
- Oil and water: Oil and water are immiscible, meaning they don't mix. They form two distinct layers, making it a heterogeneous mixture.
- Soil: Soil is a complex mixture of various minerals, organic matter, water, and air. Its composition is far from uniform, making it heterogeneous.
- Granite: This igneous rock consists of visibly different minerals like quartz, feldspar, and mica, creating a heterogeneous mixture.
- Salad: A salad is a heterogeneous mixture containing various vegetables, fruits, and potentially other components, all clearly distinguishable from each other.
- Pizza: A delicious example! The various toppings, crust, and sauce are clearly separate components, creating a heterogeneous mixture.
- Concrete: A complex mixture of cement, aggregate (sand, gravel), and water, where the different components remain largely distinct.
Distinguishing Between Homogeneous and Heterogeneous Mixtures: A Practical Approach
The key to distinguishing between homogeneous and heterogeneous mixtures lies in observing the uniformity of composition. If you can visually or microscopically identify distinct components or phases, it's a heterogeneous mixture. If the composition appears uniform throughout, it's a homogeneous mixture.
Separation Techniques for Mixtures
The methods used to separate the components of mixtures depend on whether the mixture is homogeneous or heterogeneous.
Separating Heterogeneous Mixtures:
Several techniques are used to separate the components of heterogeneous mixtures, often utilizing the differences in physical properties like size, density, and solubility:
- Filtration: This method separates solids from liquids using a porous material like filter paper. Think of filtering coffee grounds from brewed coffee.
- Decantation: Carefully pouring off a liquid from a sediment. For example, separating oil from water by allowing the denser water to settle to the bottom.
- Evaporation: This technique separates a dissolved solid from a liquid by evaporating the liquid, leaving behind the solid. Think of obtaining salt from saltwater by evaporating the water.
- Centrifugation: Using centrifugal force to separate components of different densities. Blood separation is a prime example.
- Magnetic separation: Using a magnet to separate magnetic materials from non-magnetic ones. Separating iron filings from sand is a classic example.
- Sifting: Using a sieve to separate solids of different sizes.
Separating Homogeneous Mixtures:
Separating components of a homogeneous mixture requires more sophisticated techniques as the components are intimately mixed:
- Distillation: This method is based on differences in boiling points. It's used to separate liquids with different boiling points, such as separating alcohol from water.
- Chromatography: This technique separates components based on their different affinities for a stationary and mobile phase. It's commonly used in analytical chemistry to separate complex mixtures.
- Crystallization: This method separates a solid from a liquid by allowing the solid to crystallize from a saturated solution. Salt production from saltwater is a classic example.
- Evaporation: While used for heterogeneous mixtures, evaporation can also be used for homogeneous mixtures, albeit often resulting in a change of state for at least one component.
Real-World Applications and Significance
The distinction between homogeneous and heterogeneous mixtures is not merely an academic exercise. It has significant implications across various fields:
- Material Science: Understanding the homogeneity or heterogeneity of materials is critical in designing and manufacturing materials with specific properties. For instance, the homogeneity of steel is crucial for its strength and durability.
- Environmental Science: Monitoring the composition of air and water samples often involves analyzing both homogeneous and heterogeneous mixtures to assess environmental pollution.
- Food Science: The texture and properties of many food products depend on the type of mixture involved, be it homogeneous (like a sauce) or heterogeneous (like a salad).
- Medicine: Many pharmaceutical formulations involve the careful preparation of homogeneous mixtures to ensure consistent drug delivery.
- Chemistry: Numerous chemical reactions and processes involve both homogeneous and heterogeneous mixtures, with the nature of the mixture affecting the reaction rate and outcome.
Conclusion
Differentiating between homogeneous and heterogeneous mixtures is crucial for understanding the composition and properties of various substances. This understanding is vital across diverse scientific and technological fields. By understanding the defining characteristics and employing appropriate separation techniques, we can manipulate and utilize these mixtures effectively for various applications. The examples provided throughout this guide highlight the breadth of this fundamental concept and its practical importance in our daily lives. Remember, the key lies in observing the uniformity or non-uniformity of the mixture's composition at a macroscopic level.
Latest Posts
Latest Posts
-
Movement Of Earth Around The Sun Is Called
Mar 25, 2025
-
5 Letter Words Starting With Cen
Mar 25, 2025
-
Balanced Equation For Na And H2o
Mar 25, 2025
-
What Was The Melting Point Of Water
Mar 25, 2025
-
What Is The Common Factor Of 16 And 24
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Differentiate Between Homogeneous And Heterogeneous Mixtures With Examples . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
