Balanced Equation For Na And H2o
Juapaving
Mar 25, 2025 · 6 min read
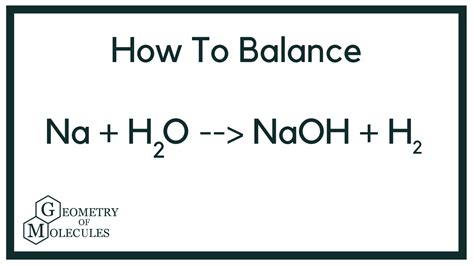
Table of Contents
The Balanced Equation for the Reaction of Sodium (Na) and Water (H₂O): A Deep Dive
The reaction between sodium (Na) and water (H₂O) is a classic example of a highly exothermic redox reaction, meaning it releases a significant amount of heat and involves the transfer of electrons. Understanding this reaction requires examining the balanced chemical equation, the underlying chemistry, safety precautions, and practical applications. This article provides a comprehensive exploration of this fascinating and important chemical process.
The Balanced Chemical Equation
The unbalanced equation for the reaction between sodium and water is:
Na + H₂O → NaOH + H₂
This equation shows the reactants (sodium and water) and the products (sodium hydroxide and hydrogen gas). However, it's crucial to balance the equation to accurately represent the stoichiometry of the reaction – the relative amounts of reactants and products involved. A balanced equation ensures that the number of atoms of each element is the same on both sides of the equation, adhering to the law of conservation of mass.
The balanced chemical equation is:
2Na + 2H₂O → 2NaOH + H₂
This balanced equation shows that two moles of sodium react with two moles of water to produce two moles of sodium hydroxide and one mole of hydrogen gas. This balance is essential for understanding the quantitative aspects of the reaction, such as predicting the amount of hydrogen gas produced from a given amount of sodium.
Understanding the Chemistry Behind the Reaction
The reaction between sodium and water is a single displacement reaction, also known as a single replacement reaction. In this type of reaction, a more reactive element displaces a less reactive element from a compound. In this case, sodium (Na), a highly reactive alkali metal, displaces hydrogen (H) from water (H₂O).
The reaction proceeds in several steps:
-
Initial Interaction: When sodium comes into contact with water, the polar water molecules attract the sodium atoms. The highly electronegative oxygen atom in water pulls the electron density away from the sodium atom.
-
Electron Transfer (Oxidation-Reduction): Sodium readily loses its single valence electron, becoming a positively charged sodium ion (Na⁺). This is an oxidation reaction because sodium loses electrons. The hydrogen atoms in water gain these electrons, reducing them to hydrogen gas (H₂). This is a reduction reaction. This electron transfer is the essence of the redox reaction.
-
Formation of Sodium Hydroxide: The sodium ions (Na⁺) then react with the hydroxide ions (OH⁻) formed from the dissociation of water to create sodium hydroxide (NaOH), a strong alkali. This is an ionic compound, meaning it's held together by electrostatic attraction between the positively charged sodium ions and the negatively charged hydroxide ions.
-
Heat Generation (Exothermic Reaction): The reaction releases a significant amount of energy in the form of heat. This is due to the strong ionic bonds formed in sodium hydroxide and the relatively weak bonds broken in water. The heat generated is often sufficient to ignite the hydrogen gas, causing a small explosion or flame.
Safety Precautions: Handling Sodium and Water
It's absolutely crucial to emphasize safety when dealing with this reaction. Sodium reacts violently with water, producing significant heat and flammable hydrogen gas. Never attempt this reaction without proper safety precautions and supervision by a qualified individual.
Here are some vital safety measures:
- Small Scale Reaction: Perform the reaction on a very small scale, using only a tiny piece of sodium (no larger than a pea).
- Appropriate Equipment: Use a heat-resistant container, such as a large beaker, and perform the reaction in a well-ventilated area or under a fume hood to dissipate the hydrogen gas.
- Eye Protection: Wear safety goggles to protect your eyes from splashes of sodium hydroxide solution.
- Gloves: Use chemical-resistant gloves to prevent skin contact with sodium and sodium hydroxide.
- Fire Safety: Have a fire extinguisher readily available in case the hydrogen gas ignites.
- Proper Disposal: Dispose of all materials according to local regulations. Sodium hydroxide is corrosive and requires careful handling and disposal.
Ignoring these precautions can lead to serious injury, including chemical burns, eye damage, and fire hazards.
Applications of the Sodium-Water Reaction
Despite the vigorous nature of the reaction, the reaction between sodium and water has several important applications:
-
Hydrogen Production: The reaction produces hydrogen gas, which is a valuable fuel source and is being increasingly studied for its role in a hydrogen economy. While not a commercially viable method for large-scale hydrogen production due to the safety concerns and cost, it demonstrates a fundamental principle.
-
Chemical Synthesis: Sodium hydroxide (NaOH), also known as lye or caustic soda, is a crucial industrial chemical with numerous applications. It's used in the production of soap, paper, textiles, and many other materials. The reaction with water offers one route, though not usually the most efficient, to produce NaOH.
-
Educational Demonstrations: The reaction is often used in chemistry demonstrations to illustrate the concepts of redox reactions, exothermic reactions, and the reactivity of alkali metals. However, these demonstrations must be conducted with extreme caution under strict safety protocols.
Further Exploring the Reaction: Factors Affecting Reaction Rate
Several factors influence the rate of the sodium-water reaction:
-
Temperature: Increasing the temperature generally increases the reaction rate. Higher temperatures provide more kinetic energy to the reactants, increasing the frequency of successful collisions leading to reaction.
-
Surface Area: A larger surface area of the sodium increases the reaction rate. A larger surface area provides more contact points for the sodium to interact with water molecules. Using finely divided sodium will dramatically increase the reaction rate compared to using a large chunk of sodium.
-
Concentration of Water: While water is typically in excess, a higher concentration of water could theoretically increase the reaction rate, increasing the frequency of collisions between sodium and water molecules. However, this effect is likely less pronounced compared to the effects of surface area and temperature.
-
Presence of Impurities: Impurities on the surface of the sodium can either catalyze or inhibit the reaction depending on their nature.
Conclusion: A Powerful and Important Reaction
The reaction between sodium and water is a fundamental chemical process demonstrating key concepts in chemistry, including redox reactions, exothermic reactions, and the reactivity of alkali metals. While the reaction is powerful and potentially dangerous, understanding its balanced equation and the chemistry involved is essential for safe handling and appreciating its applications in various fields. Always prioritize safety when working with this reaction and remember to carefully follow all appropriate safety guidelines. Further exploration of the reaction parameters can enhance your understanding of reaction kinetics and the factors that control reaction rates. This knowledge is crucial for applications ranging from industrial chemical synthesis to safer and more efficient hydrogen production.
Latest Posts
Latest Posts
-
What Is The Half Of 24
Mar 26, 2025
-
Is The Number 23 Prime Or Composite
Mar 26, 2025
-
Is Square Root Of 15 A Rational Number
Mar 26, 2025
-
Which Of These Is A Polysaccharide
Mar 26, 2025
-
What Is 7 As A Decimal
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Balanced Equation For Na And H2o . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
