What Was The Melting Point Of Water
Juapaving
Mar 25, 2025 · 5 min read
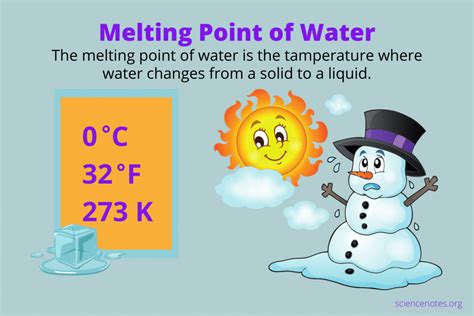
Table of Contents
What Was the Melting Point of Water? A Deep Dive into a Fundamental Property
The seemingly simple question, "What was the melting point of water?" requires a more nuanced answer than a single number. While we commonly know the melting point of water as 0°C (32°F), this is only true under specific, standard conditions. The reality is far richer, encompassing the influence of various factors that can significantly alter this fundamental property. This article will delve deep into the melting point of water, exploring its definition, the conditions affecting it, and the implications of these variations.
Defining the Melting Point
The melting point is the temperature at which a solid transitions into a liquid state. For pure substances, this transition occurs at a specific temperature under standard pressure. For water, under standard atmospheric pressure (1 atmosphere or 101.325 kPa), the melting point is indeed 0°C or 273.15 K. This is a crucial benchmark in numerous scientific fields and everyday applications.
However, the term "melting point" often overlooks the subtle yet important dynamic of the phase transition. It's not an instantaneous shift but rather a process governed by thermodynamics. The transition from ice to liquid water requires the absorption of energy, overcoming the intermolecular forces holding the water molecules in a rigid crystalline structure. This energy is known as the latent heat of fusion, representing the energy required to melt one gram of a substance at its melting point. For water, this value is relatively high, contributing to its role as a significant temperature regulator in various natural systems.
Factors Influencing the Melting Point of Water
Several factors can significantly influence the seemingly fixed melting point of water. These deviations from the standard 0°C value are crucial to understanding the behavior of water in diverse environments.
1. Pressure: A Significant Modifier
Pressure exerts a profound impact on the melting point of water. Unlike most substances, whose melting points increase with pressure, water exhibits anomalous behavior. Increasing pressure actually lowers the melting point of ice. This is due to the unique structure of ice, which has a lower density than liquid water. The application of pressure forces the less dense ice molecules closer together, favoring the denser liquid state. This explains why ice skating is possible: the pressure exerted by the skates on the ice lowers the melting point, causing a thin layer of water to form, facilitating smooth gliding.
2. Impurities: Altering the Freezing Point Depression
The presence of impurities, like dissolved salts or other solutes, depresses the freezing point of water. This is a phenomenon known as freezing point depression. The addition of solutes disrupts the ordered structure of the ice lattice, making it more difficult for the water molecules to form the solid phase. This effect is widely utilized in winter road treatments, where salt is spread to lower the freezing point of water on roads and prevent ice formation. The magnitude of the depression is proportional to the concentration of the solute.
3. Isotopic Composition: Subtle Variations
Water molecules are composed of hydrogen and oxygen atoms. However, hydrogen exists as two stable isotopes: protium (¹H) and deuterium (²H). The isotopic composition of water can slightly affect its melting point. Water enriched in deuterium (heavy water) has a higher melting point than ordinary water. This difference is relatively small but measurable and has implications for studies in various scientific fields.
4. Surface Effects: Nanoscale Influences
At the nanoscale, surface effects can play a role in altering the melting point. The melting behavior of water confined in nanoscopic pores or on surfaces differs from bulk water. The interactions of water molecules with the surrounding surface influence the energy needed for the phase transition, potentially leading to variations in the melting point. This is an area of active research with implications for nanotechnology and understanding water behavior in confined environments.
The Importance of Understanding Water's Melting Point Variations
The precise melting point of water is crucial for various scientific and practical applications. Understanding the factors that can influence this property allows for:
- Accurate predictions of natural phenomena: Knowing how pressure and impurities affect the melting point is crucial for understanding processes like glacial movements, sea ice formation, and weather patterns.
- Developing effective technologies: Applications like ice skating, road de-icing, and the design of cryogenic systems rely heavily on understanding the manipulation of water's melting point.
- Advancing scientific research: The study of water's anomalous properties, such as its melting point behavior under pressure, continues to provide valuable insights into the fundamental nature of matter and phase transitions.
- Improving industrial processes: Many industrial processes, particularly those involving water purification and freezing techniques, require precise control over temperature and pressure to optimize efficiency.
Beyond the Simple Answer: A Holistic Perspective
The seemingly simple answer to "What was the melting point of water?" — 0°C — is only a starting point. A deeper understanding reveals the complexity of this fundamental property and the myriad factors that influence it. The pressure, impurities, isotopic composition, and surface effects all play a role in shifting this melting point, leading to a more nuanced and complete picture of water's behavior. This knowledge is crucial for various scientific endeavors, technological applications, and our understanding of the natural world. The seemingly simple question opens a door to a much larger and fascinating field of study.
Exploring Further: Related Concepts and Research
Further exploration into this topic can extend into related concepts:
- Supercooled Water: This intriguing state of water exists below 0°C yet remains in its liquid phase. Understanding the conditions that allow for supercooling is crucial for scientific advancement and has applications in various technological fields.
- Ice Polymorphism: Water can exist in various crystalline forms (ice Ih, ice II, etc.), each with its own unique crystal structure and melting point. Exploring these different forms of ice provides insights into the versatile behavior of water molecules under varying conditions.
- Water's Anomalous Properties: Water exhibits numerous anomalous properties, setting it apart from other substances. Its melting point behavior is just one aspect of these anomalies, highlighting its unique and multifaceted nature.
This exploration of water's melting point showcases the significance of understanding the interplay of various factors that can modify seemingly constant properties. The quest for a single definitive answer reveals a far more dynamic and multifaceted reality, emphasizing the complexity and wonder inherent in the seemingly simple substance – water. Future research continues to deepen our understanding, promising further insights into this fundamental property and its wide-ranging implications.
Latest Posts
Latest Posts
-
What Is The Half Of 24
Mar 26, 2025
-
Is The Number 23 Prime Or Composite
Mar 26, 2025
-
Is Square Root Of 15 A Rational Number
Mar 26, 2025
-
Which Of These Is A Polysaccharide
Mar 26, 2025
-
What Is 7 As A Decimal
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Was The Melting Point Of Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
