Choose The Best Reagents To Complete The Reaction Shown Below
Juapaving
Mar 28, 2025 · 5 min read
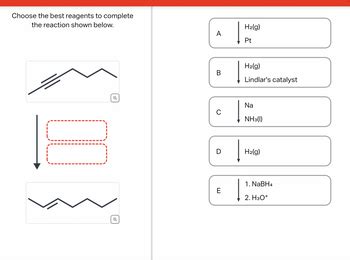
Table of Contents
Choosing the Best Reagents: A Comprehensive Guide to Reaction Optimization
Selecting the optimal reagents for a chemical reaction is crucial for achieving high yields, selectivity, and efficiency. This process is not simply about choosing the first reagent that works; it's about strategically optimizing the reaction pathway to maximize desired products and minimize unwanted side reactions. This article delves into the critical factors influencing reagent selection, focusing on how to systematically evaluate and choose the best reagents for a given transformation. We'll illustrate these principles with practical examples, emphasizing the importance of considering reaction mechanisms, solvent effects, and potential side reactions.
Understanding the Reaction: The Foundation of Reagent Selection
Before even considering specific reagents, a thorough understanding of the desired reaction mechanism is paramount. This includes identifying:
- The reaction type: Is it an addition, substitution, elimination, redox reaction, or a combination thereof? Knowing the reaction type narrows down the possibilities considerably.
- The functional groups involved: Understanding the reactivity of the functional groups present in the substrates is vital. Certain functional groups are particularly sensitive to specific reagents, leading to unwanted side reactions.
- Stereochemistry: If stereochemistry is important, then the choice of reagent must take into account its potential to influence stereoselectivity (the preference for the formation of one stereoisomer over others). This often necessitates chiral reagents or catalysts.
- The desired product: What is the target molecule, and what are its key structural features? This dictates the required transformations and constraints on reagent selection.
Case Study: The Synthesis of an Ester
Let's consider the synthesis of an ester through Fischer esterification. This reaction involves the reaction of a carboxylic acid with an alcohol in the presence of an acid catalyst. The general reaction is:
RCOOH + R'OH ⇌ RCOOR' + H₂O
The equilibrium can be shifted to favor ester formation by removing water (using a Dean-Stark apparatus, for example). However, the choice of acid catalyst significantly impacts the reaction rate and selectivity.
Evaluating Potential Reagents: Key Considerations
Once the reaction is understood, potential reagents can be evaluated based on several key factors:
- Reactivity: How readily will the reagent participate in the desired reaction? A highly reactive reagent might lead to faster reaction times but also increase the likelihood of unwanted side reactions.
- Selectivity: Does the reagent preferentially react with the desired functional group, minimizing side reactions with other functional groups in the molecule?
- Yield: What is the expected yield of the desired product using this reagent? High yields are naturally preferred, reducing the need for purification and improving overall efficiency.
- Cost and Availability: The economic feasibility of a reagent is an important practical consideration. Some reagents are expensive or difficult to obtain, which may outweigh their superior reactivity or selectivity.
- Toxicity and Safety: The environmental impact and safety of the reagent must be evaluated. Harmful or hazardous reagents should be avoided whenever possible, opting for greener alternatives.
- Ease of Purification: The resulting product mixture needs to be easily purified to isolate the desired product. This involves consideration of the solubility characteristics of both the product and by-products.
Reagent Selection for Esterification: A Deeper Dive
Returning to the esterification reaction, several acids can catalyze the reaction:
- Sulfuric Acid (H₂SO₄): A strong acid, sulfuric acid is highly effective but can also lead to sulfonation of the reactants if used in excess or under harsh conditions. It's also corrosive and requires careful handling.
- p-Toluenesulfonic Acid (TsOH): A milder acid, TsOH offers good catalytic activity with reduced risk of side reactions compared to sulfuric acid. It's also easier to remove from the reaction mixture.
- Lewis Acids (e.g., BF₃): Lewis acids can also catalyze esterification, offering different selectivity profiles depending on the substrate and reaction conditions.
Choosing between these options depends on the specific reactants, desired yield, and the tolerance for side reactions. For delicate substrates, TsOH might be preferred, while for robust substrates, H₂SO₄ might offer faster reaction times.
Optimizing Reaction Conditions: Synergistic Effects
Reagent selection is rarely isolated; it's intricately linked to reaction conditions. Factors such as temperature, solvent, concentration, and reaction time significantly influence the outcome. Optimizing these parameters alongside reagent selection leads to significant improvements in yield and selectivity.
Solvent Effects: A Critical Consideration
The choice of solvent can dramatically impact reaction rates and selectivity. Solvents can:
- Solvate reagents: Improving their solubility and reactivity.
- Stabilize intermediates: Influencing reaction pathways.
- Affect equilibrium: Shifting the reaction towards product formation.
For example, polar aprotic solvents are often preferred for reactions involving nucleophilic substitution, while non-polar solvents are better suited for reactions that involve non-polar reactants.
Temperature Control: Balancing Rate and Selectivity
Temperature plays a crucial role in reaction kinetics and thermodynamics. Higher temperatures generally increase reaction rates but can also lead to unwanted side reactions or decomposition of products. Lower temperatures can improve selectivity but may lead to slower reaction times. Careful optimization is therefore crucial to find the optimal temperature for a specific reaction.
Advanced Techniques: Expanding the Reagent Toolkit
The field of reagent development is constantly evolving, introducing new and improved reagents with enhanced reactivity, selectivity, and sustainability. These include:
- Chiral reagents: Used to achieve stereoselective synthesis.
- Organometallic reagents: Powerful tools for carbon-carbon bond formation.
- Green reagents: Designed to minimize environmental impact.
- Photoredox catalysts: Enabling new and efficient reaction pathways using light.
The selection of these advanced reagents requires a deeper understanding of their specific properties and reaction mechanisms.
Conclusion: A Systematic Approach to Reagent Selection
Choosing the best reagents for a chemical reaction is a multifaceted process that requires careful consideration of the reaction mechanism, reagent properties, reaction conditions, and potential side reactions. A systematic approach, involving a thorough understanding of the reaction and a careful evaluation of potential reagents based on their reactivity, selectivity, cost, and safety, is essential for achieving optimal results. By combining knowledge of reaction mechanisms with a strategic approach to reagent selection and reaction optimization, chemists can efficiently synthesize a wide range of molecules with high yields and selectivity. Remember that ongoing experimentation and refinement are key to achieving the best results. The journey to finding the "best" reagent often involves iterative testing and optimization of reaction conditions. This systematic approach not only leads to improved yields and selectivity but also contributes to the development of more sustainable and efficient chemical processes.
Latest Posts
Latest Posts
-
The Rate Of Change Of Velocity Is Called
Mar 31, 2025
-
5 Levels Of Organization In An Ecosystem
Mar 31, 2025
-
Would Silver React With Dilute Sulfuric Acid
Mar 31, 2025
-
How Many Factors Does A Composite Number Have
Mar 31, 2025
-
What Is 4 The Square Root Of
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Choose The Best Reagents To Complete The Reaction Shown Below . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
