Can Pure Substances Be Separated By Physical Means
Juapaving
Mar 25, 2025 · 5 min read
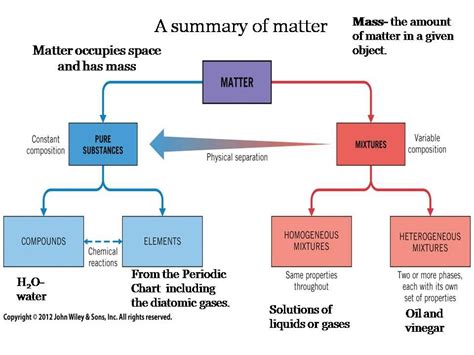
Table of Contents
Can Pure Substances Be Separated by Physical Means?
The question of whether pure substances can be separated by physical means is a fundamental concept in chemistry. The answer, in short, is no. A pure substance, by definition, is a form of matter that has a constant composition and properties throughout the sample. This contrasts with mixtures, which are composed of two or more substances that are not chemically bonded and can be separated by physical means. Let's delve deeper into this crucial distinction and explore the various methods used to separate mixtures, highlighting why these methods are ineffective for pure substances.
Understanding Pure Substances and Mixtures
Before we explore separation techniques, it's crucial to define our terms.
Pure Substances: The Building Blocks of Matter
A pure substance is a single type of matter with a fixed chemical composition. It possesses consistent physical and chemical properties throughout the sample. This means that no matter where you take a sample from a pure substance (e.g., a gold bar), its properties—like melting point, boiling point, density, and reactivity—will remain the same. Pure substances can be further categorized into:
-
Elements: These are substances composed of only one type of atom. Examples include oxygen (O), gold (Au), and iron (Fe). They cannot be broken down into simpler substances by chemical means.
-
Compounds: These are substances formed when two or more elements chemically combine in fixed proportions. Water (H₂O), table salt (NaCl), and carbon dioxide (CO₂) are examples of compounds. They can be broken down into their constituent elements through chemical processes, but not by physical ones.
Mixtures: A Blend of Substances
Mixtures, unlike pure substances, are composed of two or more substances that are physically combined but not chemically bonded. The components of a mixture retain their individual properties, and their proportions can vary. There are two main types of mixtures:
-
Homogeneous Mixtures: These mixtures have a uniform composition throughout. Examples include saltwater, air, and sugar dissolved in water. The individual components are not visually distinguishable.
-
Heterogeneous Mixtures: These mixtures have a non-uniform composition. The individual components are visually distinguishable. Examples include sand and water, oil and water, and a salad.
Physical Separation Techniques for Mixtures
A wide array of physical methods can separate the components of a mixture, exploiting differences in their physical properties. These methods are ineffective for separating pure substances because, by definition, pure substances lack the physical variations that these techniques target.
1. Filtration: Separating Solids from Liquids
Filtration utilizes a porous material (like filter paper) to separate a solid from a liquid. The liquid passes through the pores, while the solid particles are trapped. This works because of the difference in particle size between the solid and the liquid. This method is ideal for separating heterogeneous mixtures like sand and water.
2. Decantation: Separating Liquids of Different Densities
Decantation is a simple technique used to separate immiscible liquids (liquids that don't mix) based on their density difference. The less dense liquid is carefully poured off, leaving the denser liquid behind. Oil and water separation is a classic example of decantation.
3. Evaporation: Separating a Solute from a Solvent
Evaporation separates a dissolved solid (solute) from a liquid (solvent) by heating the solution. The solvent evaporates, leaving the solid behind. This is commonly used to obtain salt from saltwater.
4. Distillation: Separating Liquids with Different Boiling Points
Distillation is a more sophisticated method used to separate liquids with different boiling points. The mixture is heated, and the component with the lower boiling point vaporizes first. The vapor is then condensed back into a liquid and collected separately. This process is used to purify water and separate alcohol from fermented mixtures.
5. Chromatography: Separating Components based on Affinity
Chromatography is a powerful technique used to separate mixtures based on the different affinities of the components for a stationary phase (e.g., paper, silica gel) and a mobile phase (e.g., a solvent). The components move at different rates through the stationary phase, resulting in separation. This technique is widely used in analytical chemistry and biochemistry.
6. Magnetism: Separating Magnetic from Non-Magnetic Materials
This simple technique uses a magnet to separate magnetic materials (like iron) from non-magnetic ones. It exploits the difference in magnetic susceptibility between the components.
7. Centrifugation: Separating Components based on Density
Centrifugation uses centrifugal force to separate components with different densities. The denser components settle at the bottom, while the lighter ones remain at the top. This is commonly used in laboratories to separate blood components.
8. Sublimation: Separating Solids with Different Sublimation Points
Sublimation is the transition of a substance from the solid phase directly to the gaseous phase, without passing through the liquid phase. This technique can separate components with different sublimation points. A classic example is separating iodine from a mixture.
Why Physical Methods Fail with Pure Substances
All the physical separation methods described above rely on differences in the physical properties of the components of a mixture. Pure substances, by definition, have uniform physical properties throughout the sample. There's no difference in boiling point, density, solubility, or any other physical property to exploit for separation.
Attempting to use a physical separation technique on a pure substance will simply result in obtaining the same pure substance. For instance, trying to distill pure water will yield… pure water. The process won't separate it into anything else because there's nothing else to separate.
Chemical Separation Techniques: Breaking Down Compounds
While physical methods are ineffective for separating pure substances, chemical methods can be used to break down compounds into their constituent elements. These methods involve chemical reactions that alter the chemical composition of the substance. Examples include electrolysis (used to decompose water into hydrogen and oxygen) and chemical reactions that break down more complex molecules.
Conclusion: The Inseparability of Pure Substances
In summary, pure substances cannot be separated by physical means. The very definition of a pure substance implies uniformity in its composition and properties. Physical separation techniques exploit differences in physical properties, which are absent in pure substances. To break down pure substances, particularly compounds, chemical methods are necessary. Understanding this fundamental distinction is crucial in chemistry and various related scientific fields. The ability to separate mixtures efficiently is vital in many industrial processes and analytical techniques, while the understanding of the inherent homogeneity of pure substances underpins numerous chemical principles and applications.
Latest Posts
Latest Posts
-
Binomial System Of Nomenclature Was Proposed By
Mar 28, 2025
-
What Is 1 625 In Fraction Form
Mar 28, 2025
-
What Is The Square Root Of One
Mar 28, 2025
-
How Many Lines Of Symmetry Does An Isosceles Trapezium Have
Mar 28, 2025
-
What Is All The Factors Of 49
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Can Pure Substances Be Separated By Physical Means . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
