Bronsted Lowry Acid Vs Lewis Acid
Juapaving
Mar 09, 2025 · 6 min read
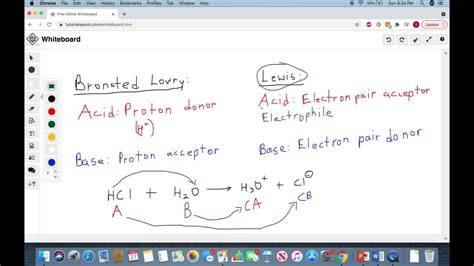
Table of Contents
Brønsted-Lowry Acid vs. Lewis Acid: A Comprehensive Comparison
Understanding the concepts of acids and bases is fundamental to chemistry. While numerous definitions exist, the Brønsted-Lowry and Lewis definitions are the most commonly used and offer slightly different perspectives on these fundamental chemical species. This comprehensive guide delves into the distinctions between Brønsted-Lowry and Lewis acids, exploring their definitions, examples, and the overlaps and divergences between them.
Defining Brønsted-Lowry Acids and Bases
The Brønsted-Lowry theory, proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923, defines acids and bases based on proton transfer.
-
Brønsted-Lowry Acid: A Brønsted-Lowry acid is a substance that donates a proton (H⁺) to another substance. It's a proton donor. The key here is the ability to release a hydrogen ion.
-
Brønsted-Lowry Base: A Brønsted-Lowry base is a substance that accepts a proton (H⁺) from another substance. It's a proton acceptor. This usually involves a lone pair of electrons that can bond with the proton.
Example: Consider the reaction between hydrochloric acid (HCl) and water (H₂O):
HCl + H₂O ⇌ H₃O⁺ + Cl⁻
In this reaction, HCl acts as a Brønsted-Lowry acid because it donates a proton to H₂O. H₂O acts as a Brønsted-Lowry base because it accepts the proton. The resulting H₃O⁺ (hydronium ion) is the conjugate acid of H₂O, and Cl⁻ is the conjugate base of HCl. The reaction is an equilibrium, meaning the reverse reaction can also occur.
Key Characteristics of Brønsted-Lowry Acids:
- Proton donation: The defining characteristic.
- Presence of acidic hydrogen: Usually involves a hydrogen atom bonded to an electronegative atom (like oxygen or chlorine).
- Conjugate base formation: Upon donating a proton, the acid forms its conjugate base.
- Strength depends on ease of proton donation: Strong acids readily donate protons, while weak acids donate protons less readily.
Defining Lewis Acids and Bases
Gilbert N. Lewis proposed a broader definition of acids and bases in 1923, focusing on electron pair donation and acceptance. This definition encompasses a wider range of reactions than the Brønsted-Lowry definition.
-
Lewis Acid: A Lewis acid is a substance that accepts an electron pair. It's an electron pair acceptor. This often involves an atom with an incomplete octet or a vacant orbital that can accept electrons.
-
Lewis Base: A Lewis base is a substance that donates an electron pair. It's an electron pair donor. This usually involves an atom with a lone pair of electrons.
Example: Consider the reaction between boron trifluoride (BF₃) and ammonia (NH₃):
BF₃ + NH₃ → F₃B-NH₃
In this reaction, BF₃ acts as a Lewis acid because it accepts an electron pair from NH₃. NH₃ acts as a Lewis base because it donates an electron pair to BF₃. BF₃ has an incomplete octet (only 6 electrons around boron), making it electron-deficient and able to accept an electron pair.
Key Characteristics of Lewis Acids:
- Electron pair acceptance: The defining characteristic.
- Electron deficiency: Often, but not always, involves an atom with an incomplete octet.
- Presence of vacant orbitals: Can accept electron pairs into vacant orbitals.
- Variety of chemical species: Includes metal ions, molecules with polar bonds, and many others.
Comparing Brønsted-Lowry and Lewis Acid-Base Theories
While seemingly different, the Lewis definition is a generalization of the Brønsted-Lowry definition. All Brønsted-Lowry acids and bases are also Lewis acids and bases, but not all Lewis acids and bases are Brønsted-Lowry acids and bases.
Overlaps:
- Proton transfer reactions: Reactions involving proton transfer are encompassed by both theories. The proton (H⁺) is acting as a Lewis acid (electron pair acceptor) and the base as a Lewis base (electron pair donor).
Divergences:
-
Scope: The Lewis definition is broader. It includes reactions that do not involve proton transfer, such as the reaction between BF₃ and NH₃ (mentioned above). The Brønsted-Lowry theory is limited to reactions involving proton transfer.
-
Species included: The Lewis theory includes a wider range of substances as acids and bases. Many metal ions, for instance, can act as Lewis acids, even though they don't donate protons. Similarly, many molecules without acidic hydrogens can still act as Lewis bases.
Examples to Illustrate the Differences
Let's look at a few examples to further clarify the differences:
1. HCl reacting with H₂O (Brønsted-Lowry and Lewis):
This reaction, as discussed earlier, fits both definitions. HCl is a Brønsted-Lowry acid (donates a proton) and a Lewis acid (accepts an electron pair from H₂O). H₂O is a Brønsted-Lowry base (accepts a proton) and a Lewis base (donates an electron pair to H⁺).
2. BF₃ reacting with NH₃ (Lewis only):
This reaction is a classic example of a Lewis acid-base reaction that doesn't involve proton transfer. BF₃ acts as a Lewis acid, and NH₃ acts as a Lewis base. There's no proton donation or acceptance.
3. AlCl₃ reacting with Cl⁻ (Lewis only):
Aluminum chloride (AlCl₃) acts as a Lewis acid by accepting an electron pair from the chloride ion (Cl⁻), which acts as a Lewis base. This reaction doesn't involve any proton transfer.
Applications and Significance
Understanding both Brønsted-Lowry and Lewis acid-base theories is crucial in various fields:
-
Organic Chemistry: These theories are fundamental in understanding reaction mechanisms, particularly those involving nucleophilic and electrophilic attacks. Lewis acids are frequently used as catalysts in organic reactions.
-
Inorganic Chemistry: The Lewis theory is vital for understanding the behavior of metal complexes and coordination compounds. Many metal ions act as Lewis acids, forming complexes with Lewis bases (ligands).
-
Biochemistry: Many biological processes involve acid-base reactions. Enzymes often utilize Lewis acid-base interactions for catalysis. The interaction between metal ions and biomolecules is frequently explained using the Lewis theory.
-
Analytical Chemistry: Acid-base titrations are a common analytical technique reliant on the Brønsted-Lowry theory, while understanding Lewis acidity is crucial in many analytical methods.
Strong vs. Weak Acids and Bases: A Nuance
The strength of an acid or base can be defined differently depending on whether you're using the Brønsted-Lowry or Lewis definitions. In the Brønsted-Lowry context, strength refers to the ease of proton donation or acceptance. In the Lewis context, it relates to the strength of the electron pair donation or acceptance. Strong Lewis acids readily accept electron pairs, while weak Lewis acids accept them less readily. The same logic applies to Lewis bases. It's important to note that the terms "strong" and "weak" are often used in the context of Brønsted-Lowry acids and bases, but the concept can be extended to Lewis acids and bases as well.
Conclusion
The Brønsted-Lowry and Lewis theories provide different but complementary perspectives on acids and bases. The Brønsted-Lowry theory is simpler and focuses on proton transfer, while the Lewis theory is broader and focuses on electron pair donation and acceptance. Understanding both theories is critical for a complete grasp of acid-base chemistry and its applications across various scientific disciplines. While the Brønsted-Lowry theory is a subset of the Lewis theory, both offer valuable insights into chemical reactivity and are essential tools for understanding a vast range of chemical phenomena. The choice of which definition to use depends on the specific context and the types of reactions being considered. However, appreciating the broader scope of the Lewis theory is key to understanding many complex reactions that don't involve straightforward proton transfers.
Latest Posts
Latest Posts
-
Characteristic Polynomial Of A 3x3 Matrix
Mar 09, 2025
-
How Do You Spell The Number 30
Mar 09, 2025
-
Is Nitrogen Metal Nonmetal Or Metalloid
Mar 09, 2025
-
How To Find Square Root Of Fraction
Mar 09, 2025
-
What Are The Factors Of 53
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about Bronsted Lowry Acid Vs Lewis Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
