Boiling Point Of Water On Kelvin Scale
Juapaving
Mar 21, 2025 · 5 min read
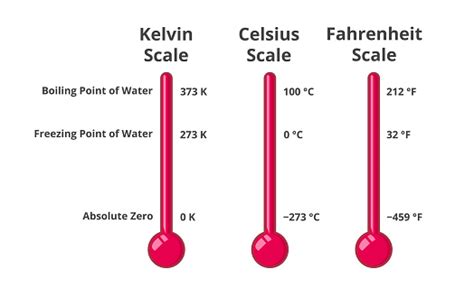
Table of Contents
Boiling Point of Water on the Kelvin Scale: A Deep Dive
The boiling point of water is a fundamental concept in science and everyday life. While we commonly express it in Celsius or Fahrenheit, understanding its equivalent on the Kelvin scale provides a deeper appreciation for its thermodynamic significance. This article will explore the boiling point of water on the Kelvin scale, delving into its definition, the underlying physics, applications, and factors that can influence this crucial value.
What is the Kelvin Scale?
The Kelvin scale, named after Lord Kelvin (William Thomson), is an absolute thermodynamic temperature scale. Unlike Celsius and Fahrenheit, which are relative scales with arbitrary zero points, the Kelvin scale starts at absolute zero – the theoretical temperature at which all molecular motion ceases. This makes it a particularly useful scale for scientific purposes, as many physical laws and relationships are more elegantly expressed in terms of absolute temperature.
Key characteristics of the Kelvin scale:
- Absolute Zero: 0 Kelvin (0 K) represents absolute zero, approximately -273.15°C or -459.67°F.
- Increments: The size of a Kelvin degree is the same as that of a Celsius degree. This means a change of 1 K is equivalent to a change of 1°C.
- No negative values: Temperatures on the Kelvin scale are always positive, reflecting the absolute nature of the scale.
The Boiling Point of Water: A Definition
The boiling point of a liquid is the temperature at which its vapor pressure equals the surrounding atmospheric pressure. At this point, bubbles of vapor form within the liquid and rise to the surface, causing the liquid to boil. The boiling point of water, under standard atmospheric pressure (1 atmosphere or 101.325 kPa), is 100°C.
Converting Celsius to Kelvin
Converting between Celsius and Kelvin is straightforward. To convert a temperature from Celsius to Kelvin, simply add 273.15:
K = °C + 273.15
Therefore, the boiling point of water in Kelvin is:
K = 100°C + 273.15 = 373.15 K
The Significance of 373.15 K
The value of 373.15 K is not just a simple conversion; it holds significant implications in various fields:
- Thermodynamics: This temperature represents a specific point on the thermodynamic temperature scale, enabling precise calculations involving enthalpy, entropy, and Gibbs free energy. Understanding this boiling point is crucial for calculations related to phase transitions, heat transfer, and other thermodynamic processes.
- Chemistry: Many chemical reactions and processes are temperature-dependent. Knowing the boiling point of water in Kelvin allows for accurate control and prediction of reaction rates and equilibrium conditions in experiments and industrial applications.
- Physics: The boiling point is related to the intermolecular forces within water molecules. At 373.15 K, the kinetic energy of water molecules overcomes these forces, enabling the transition from liquid to gas. This concept is central to understanding phase transitions and the behavior of matter at different temperatures.
- Engineering: In various engineering applications, particularly in thermal engineering and power generation, accurate knowledge of the boiling point of water in Kelvin is crucial for designing efficient and safe systems. Examples include steam turbines, cooling systems, and heat exchangers.
Factors Affecting the Boiling Point of Water
While 373.15 K is the standard boiling point of water, several factors can influence this value:
1. Atmospheric Pressure
This is the most significant factor. At higher altitudes, where atmospheric pressure is lower, water boils at a lower temperature. Conversely, at higher pressures, the boiling point increases. This is why a pressure cooker cooks food faster – the increased pressure raises the boiling point of water, allowing higher temperatures to be reached.
2. Impurities
Dissolved impurities in water can slightly alter its boiling point. Generally, the presence of dissolved substances elevates the boiling point, a phenomenon known as boiling point elevation. This effect is more pronounced with higher concentrations of solutes.
3. Isotopic Composition
Water molecules composed of different isotopes of hydrogen and oxygen (e.g., deuterium instead of protium) will have slightly different boiling points. Heavy water (D₂O) boils at a higher temperature than ordinary water (H₂O).
Applications of Understanding the Boiling Point
The understanding of water's boiling point at 373.15 K is crucial across a multitude of disciplines and applications:
- Cooking: Understanding how altitude affects the boiling point is essential for adjusting cooking times and methods.
- Steam Power Generation: Steam turbines rely on the boiling of water to generate power. Precise knowledge of the boiling point under varying pressures is vital for efficient operation.
- Sterilization: Boiling water is a common method for sterilization, as the high temperature kills many microorganisms. Understanding the boiling point ensures effective sterilization.
- Chemical Processes: Many industrial chemical processes utilize water at or near its boiling point, requiring precise temperature control.
- Refrigeration: The boiling point of refrigerants is a key parameter in refrigeration systems.
- Meteorology: The boiling point of water is crucial in understanding atmospheric processes such as cloud formation and precipitation.
Beyond the Boiling Point: Phase Transitions
Understanding the boiling point of water at 373.15 K is just one piece of the puzzle when it comes to the behavior of water. Water also undergoes other phase transitions:
- Melting Point: The temperature at which ice melts into water is 0°C or 273.15 K (at standard pressure).
- Freezing Point: The temperature at which water freezes into ice is also 0°C or 273.15 K (at standard pressure). Note that the freezing and melting points are the same at standard pressure.
- Sublimation: Under certain conditions, ice can transition directly from solid to gas (water vapor) without passing through the liquid phase. This process is called sublimation.
Conclusion: The Importance of 373.15 K
The boiling point of water at 373.15 K on the Kelvin scale is not merely a numerical value; it represents a fundamental physical constant with profound implications across various scientific disciplines and everyday applications. Understanding its significance, the factors that influence it, and its role in phase transitions is crucial for anyone seeking a deeper understanding of the physical world around us. From cooking to power generation to chemical engineering, the knowledge of this crucial temperature remains indispensable. The absolute nature of the Kelvin scale makes it particularly suitable for precise scientific work and complex calculations involving thermodynamic properties and chemical reactions. This comprehensive understanding extends beyond simple conversion and emphasizes the importance of this temperature in countless processes and phenomena.
Latest Posts
Latest Posts
-
Moment Of Inertia Of Quarter Circle
Mar 28, 2025
-
What Are Common Multiples Of 6 And 8
Mar 28, 2025
-
State Newtons Law Of Universal Gravitation In Words
Mar 28, 2025
-
Is 6 A Factor Of 84
Mar 28, 2025
-
What Is The Lcm Of 2 And 11
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Boiling Point Of Water On Kelvin Scale . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
