Blood Is A Mixture Or Compound
Juapaving
Mar 24, 2025 · 5 min read
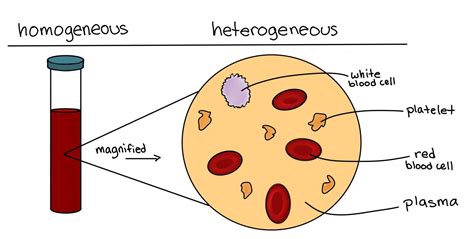
Table of Contents
Blood: A Mixture, Not a Compound – Delving into the Composition of Life's River
Blood, the crimson river coursing through our veins, is often perceived as a singular entity. However, a closer look reveals a complex composition, far from a simple compound. This article will delve deep into the intricate nature of blood, demonstrating definitively why it's classified as a mixture, rather than a compound. We'll explore its components, their individual properties, and how their combination creates a dynamic and essential fluid vital for life.
Understanding the Difference: Mixture vs. Compound
Before diving into the specifics of blood, let's establish a clear understanding of the fundamental difference between a mixture and a compound.
-
Compound: A compound is a substance formed when two or more chemical elements are chemically bonded together. These bonds create a new substance with properties distinctly different from its constituent elements. For example, water (H₂O) is a compound formed from the bonding of hydrogen and oxygen. The properties of water are completely different from the properties of hydrogen gas and oxygen gas. The ratio of elements in a compound is always fixed and defined by its chemical formula.
-
Mixture: A mixture is a substance composed of two or more components that are physically combined but not chemically bonded. The components retain their individual chemical properties, and their proportions can vary. A salad, for instance, is a mixture of various vegetables – each retaining its unique characteristics. Similarly, air is a mixture of various gases like nitrogen, oxygen, and carbon dioxide.
The Heterogeneous Nature of Blood: Evidence for a Mixture
Blood's classification as a mixture stems from its heterogeneous nature. This means its components are not uniformly distributed throughout the solution. You can readily observe this heterogeneity with the naked eye, or with a simple microscope.
1. Cellular Components: A Diverse Population
Blood isn't a homogenous solution; instead, it's a suspension of various cellular components within a fluid matrix. These cells include:
-
Red Blood Cells (Erythrocytes): These biconcave discs are responsible for carrying oxygen throughout the body. Their primary component is hemoglobin, an iron-containing protein that binds to oxygen. The number of red blood cells can vary depending on factors such as altitude and overall health.
-
White Blood Cells (Leukocytes): These are the body's defense cells, combating infections and foreign invaders. There are several types of white blood cells, each with specialized functions. Their concentration changes in response to illness and infection.
-
Platelets (Thrombocytes): These small, irregular cell fragments play a crucial role in blood clotting, preventing excessive bleeding. Their numbers also fluctuate based on the body's needs.
The presence of these distinct cell types, each with its own structure and function, immediately indicates that blood is a mixture, not a compound. These cells are not chemically bonded to each other or the fluid portion of blood.
2. Plasma: The Fluid Matrix
The fluid portion of blood, known as plasma, is a complex solution itself. It's primarily composed of water (approximately 90%), but it also contains a variety of dissolved substances, including:
-
Proteins: Plasma proteins, such as albumin, globulins, and fibrinogen, play crucial roles in maintaining osmotic pressure, transporting molecules, and blood clotting.
-
Electrolytes: Essential ions like sodium, potassium, calcium, and chloride are vital for maintaining fluid balance, nerve function, and muscle contraction. Their concentrations are meticulously regulated.
-
Nutrients: Glucose, amino acids, lipids, and vitamins are transported throughout the body via plasma. Their levels vary depending on dietary intake and metabolic activity.
-
Waste Products: Plasma carries metabolic waste products, such as urea and creatinine, to the kidneys for excretion.
The varying concentrations of these dissolved substances demonstrate that plasma, and consequently blood, is a mixture. These components aren't chemically bonded; rather, they're dissolved or suspended in the water-based solution.
3. Variable Composition: A Defining Feature of Mixtures
One of the most compelling arguments for blood being a mixture is its variable composition. The relative proportions of different components in blood can fluctuate significantly depending on various factors, including:
-
Diet: Nutrient levels in plasma directly reflect dietary intake.
-
Hydration: Plasma volume changes depending on fluid consumption.
-
Health Status: Infections, diseases, and hormonal imbalances can drastically alter the cellular composition of blood.
-
Altitude: Red blood cell count increases at high altitudes to compensate for lower oxygen levels.
This variability in composition is a hallmark of mixtures. In a compound, the ratio of constituent elements remains constant; blood's dynamic nature contradicts this characteristic.
Further Evidence Against Blood Being a Compound
The chemical properties of blood's components also support its classification as a mixture. The individual components retain their characteristic properties even when combined in blood. For instance:
-
Hemoglobin's oxygen-carrying capacity: Hemoglobin's ability to bind and release oxygen isn't altered by its presence within red blood cells or plasma.
-
Enzyme activity: Various enzymes present in plasma retain their catalytic activity, unaffected by the presence of other plasma components.
-
Electrolyte balance: The individual properties of electrolytes—their charge, their ability to conduct electricity, etc.—remain unchanged in blood.
If blood were a compound, these individual components would likely lose or significantly alter their properties during the hypothetical chemical bonding process. The preservation of individual component properties strengthens the evidence that blood is a mixture.
The Significance of Blood's Mixture Nature
The fact that blood is a mixture rather than a compound is fundamentally important to its biological function. This heterogeneity allows for:
-
Efficient Transport: The diverse components of blood can effectively transport oxygen, nutrients, hormones, and waste products throughout the body.
-
Immune Response: The presence of various white blood cells allows for a tailored immune response to different pathogens.
-
Homeostasis: The intricate balance of plasma components helps maintain a stable internal environment.
-
Adaptability: The variability in blood composition allows it to adapt to changing physiological needs and environmental conditions.
Conclusion: A Complex Mixture, Essential for Life
In conclusion, compelling evidence solidifies blood's classification as a heterogeneous mixture, not a compound. Its diverse cellular components, variable composition, and the retention of individual component properties all point to this conclusion. Understanding blood's complex composition is crucial for appreciating its vital role in sustaining life. The intricate interplay of its various components allows for efficient transport, immune defense, and the maintenance of a stable internal environment. This remarkable fluid, a complex mixture, is truly the lifeblood of our bodies.
Latest Posts
Latest Posts
-
How Does Temperature Relate To Kinetic Energy
Mar 26, 2025
-
The Flow Of Electrons Is Called
Mar 26, 2025
-
The Movement Of Materials From Low To High Concentration
Mar 26, 2025
-
How Many Thousands Are In A Billion
Mar 26, 2025
-
Protons Neutrons And Electrons Of Potassium
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Blood Is A Mixture Or Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
