Protons Neutrons And Electrons Of Potassium
Juapaving
Mar 26, 2025 · 5 min read
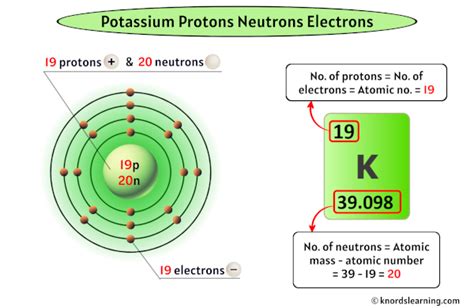
Table of Contents
Protons, Neutrons, and Electrons of Potassium: A Deep Dive into the Atom
Potassium, a crucial element for life, plays a vital role in numerous biological processes. Understanding its atomic structure, particularly the number of protons, neutrons, and electrons, is fundamental to grasping its chemical behavior and biological significance. This article delves into the fascinating world of potassium's subatomic particles, exploring their arrangement, properties, and the implications of this structure.
Understanding Potassium's Place in the Periodic Table
Potassium (K), atomic number 19, resides in Group 1 (alkali metals) of the periodic table. Its position dictates its chemical properties, primarily its highly reactive nature due to its single valence electron. This electron is easily lost, forming a +1 ion (K⁺), which participates extensively in biological processes. The arrangement of protons, neutrons, and electrons is intrinsically linked to this reactivity and the element's overall characteristics.
The Subatomic Trio: Protons, Neutrons, and Electrons
Let's dissect the three fundamental subatomic particles that constitute a potassium atom:
Protons: The Defining Characteristic
- What they are: Positively charged particles residing in the atom's nucleus.
- Number in Potassium: Potassium's atomic number is 19, meaning every potassium atom possesses 19 protons. This number is immutable; it defines potassium as potassium and differentiates it from all other elements. Changing the proton count transforms the element entirely.
- Role in the Atom: Protons determine the element's identity and its chemical behavior. They contribute significantly to the atom's mass and positive charge.
- Mass: Approximately 1 atomic mass unit (amu).
Neutrons: The Nuclear Partners
- What they are: Neutrally charged particles also located within the atom's nucleus. Unlike protons, the number of neutrons can vary within an element, leading to isotopes.
- Number in Potassium: Potassium has several naturally occurring isotopes. The most abundant is potassium-39 (³⁹K), containing 20 neutrons. Other less abundant isotopes include potassium-40 (⁴⁰K) with 21 neutrons and potassium-41 (⁴¹K) with 22 neutrons. These isotopes differ only in their neutron count, not their proton count.
- Role in the Atom: Neutrons contribute to the atom's mass and nuclear stability. The neutron-to-proton ratio significantly influences an atom's stability and its susceptibility to radioactive decay. In the case of potassium-40, this isotope is weakly radioactive, undergoing beta decay.
- Mass: Approximately 1 amu.
Electrons: The Orbital Dancers
- What they are: Negatively charged particles orbiting the nucleus in electron shells or energy levels.
- Number in Potassium: In a neutral potassium atom, the number of electrons equals the number of protons, ensuring a net charge of zero. Therefore, a neutral potassium atom possesses 19 electrons.
- Role in the Atom: Electrons determine the atom's chemical reactivity. The outermost electron shell (valence shell) houses the valence electrons, which are involved in chemical bonding. Potassium's single valence electron readily participates in ionic bonding, readily losing this electron to achieve a stable octet configuration.
- Mass: Negligible compared to protons and neutrons.
Isotopes of Potassium: A Closer Look
The existence of potassium isotopes highlights the variability in neutron number within the same element. Understanding isotopes is crucial for several reasons:
- Radioactive Decay: Potassium-40 (⁴⁰K) is a naturally occurring radioactive isotope. Its radioactive decay contributes to the Earth's internal heat and is used in radiometric dating.
- Biological Significance: The different isotopes of potassium behave similarly chemically, but their slightly varying masses can be exploited in scientific techniques like isotopic tracing.
- Nuclear Stability: The neutron-to-proton ratio influences an isotope's stability. Potassium-39 and Potassium-41 are stable isotopes, while Potassium-40 is radioactive.
Potassium's Role in Biology: The Importance of Ions
The biological significance of potassium primarily stems from its ionic form, K⁺. This positively charged ion plays critical roles in:
- Maintaining Osmotic Balance: Potassium ions contribute significantly to the osmotic pressure within cells, regulating the movement of water and other solutes.
- Nerve Impulse Transmission: Potassium ions are crucial for generating and transmitting nerve impulses. The movement of potassium ions across cell membranes creates the electrical potential necessary for nerve signal propagation.
- Muscle Contraction: Similar to nerve impulse transmission, potassium ions are essential for muscle contraction. The interplay of potassium and other ions like sodium and calcium facilitates muscle fiber contraction and relaxation.
- Enzyme Activation: Potassium ions act as cofactors or activators for several enzymes involved in crucial metabolic processes.
- Plant Physiology: Potassium is vital for plant growth and development, playing a critical role in photosynthesis, enzyme activation, and water regulation.
Applications of Potassium and its Isotopes
The properties of potassium and its isotopes have led to applications in various fields:
- Agriculture: Potassium fertilizers are essential for crop production, supplying potassium to plants to boost growth and yield.
- Medicine: Potassium salts are used in medical treatments, particularly for electrolyte imbalances. Potassium supplements are prescribed when potassium levels are low.
- Industry: Potassium compounds find use in various industrial applications, such as the production of soap, glass, and fertilizers.
- Scientific Research: Potassium-40's radioactive decay is utilized in radiometric dating techniques to determine the age of geological formations and artifacts. Isotopic tracing techniques employ potassium isotopes to study metabolic processes in living organisms.
Conclusion: The Significance of Understanding Potassium's Atomic Structure
Understanding the number of protons, neutrons, and electrons in a potassium atom is crucial for comprehending its chemical properties, biological roles, and applications. The 19 protons define it as potassium, while the variable neutron count results in isotopes with slightly differing properties. The readily available valence electron makes it highly reactive, leading to the formation of K⁺ ions, which are essential for numerous biological processes. The exploration of potassium's atomic structure provides a foundation for understanding its widespread importance in the natural world and its diverse applications in various fields. Further research continues to unveil new facets of this vital element's properties and significance. The fundamental understanding of its subatomic composition remains a cornerstone of this ongoing exploration.
Latest Posts
Latest Posts
-
The Original Three Components Of The Cell Theory Are That
Mar 29, 2025
-
Give The Temperature And Pressure At Stp
Mar 29, 2025
-
Give The Ground State Electron Configuration For Pb
Mar 29, 2025
-
Worksheet On Simple Compound And Complex Sentences With Answers
Mar 29, 2025
-
Lowest Common Multiple Of 6 8 And 9
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Protons Neutrons And Electrons Of Potassium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
