How Does Temperature Relate To Kinetic Energy
Juapaving
Mar 26, 2025 · 5 min read
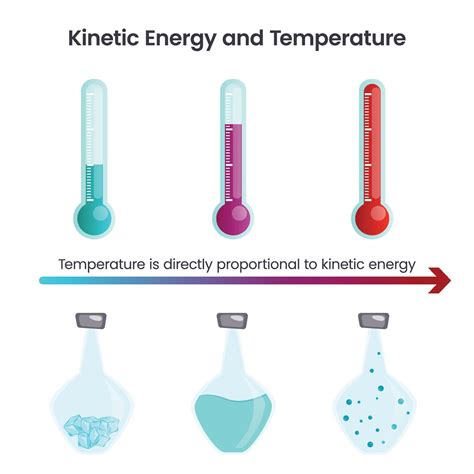
Table of Contents
How Does Temperature Relate to Kinetic Energy? A Deep Dive
Temperature and kinetic energy are intrinsically linked, forming a cornerstone of thermodynamics and our understanding of the physical world. While seemingly simple at first glance, the relationship between these two concepts reveals a rich tapestry of physical phenomena, impacting everything from the behavior of gases to the design of efficient engines. This article delves deep into the connection, exploring the microscopic world of atoms and molecules to explain the macroscopic properties we observe.
The Microscopic Dance: Atoms and Molecules in Motion
At the heart of the temperature-kinetic energy relationship lies the ceaseless motion of atoms and molecules. Even in seemingly stationary objects, these fundamental particles are in constant motion – vibrating, rotating, and translating. This constant movement is what we refer to as kinetic energy, the energy of motion. The faster the particles move, the greater their kinetic energy.
Kinetic Energy: A Quantitative Look
Kinetic energy (KE) is mathematically expressed as:
KE = 1/2 * m * v²
Where:
- m represents the mass of the particle.
- v represents the velocity of the particle.
This simple equation highlights a crucial point: kinetic energy is directly proportional to the square of the velocity. A small increase in velocity leads to a much larger increase in kinetic energy. This has significant implications for the behavior of matter at different temperatures.
Temperature: A Measure of Average Kinetic Energy
Temperature isn't a direct measure of the kinetic energy of individual particles. Instead, temperature is a measure of the average kinetic energy of the particles within a system. This means that temperature reflects the overall energy distribution among the particles.
The Importance of "Average": A Statistical Perspective
Consider a gas contained in a container. The individual molecules within the gas possess a wide range of velocities, colliding with each other and the container walls. Some molecules will be moving incredibly fast, while others will be moving relatively slowly. Temperature, however, reflects the average kinetic energy of this entire ensemble of molecules.
Absolute Zero: The Point of No Motion
The concept of average kinetic energy allows us to define absolute zero, the theoretical lowest possible temperature. At absolute zero (0 Kelvin or -273.15°C), the average kinetic energy of the particles in a system reaches its minimum possible value – theoretically zero. However, it's crucial to note that even at absolute zero, particles still possess some residual vibrational energy due to quantum mechanical effects, preventing them from becoming completely motionless.
The Relationship in Different States of Matter
The relationship between temperature and kinetic energy manifests differently across the three fundamental states of matter: solids, liquids, and gases.
Solids: Restricted Motion, Vibrational Energy
In solids, the atoms and molecules are tightly bound together in a fixed lattice structure. Their motion is primarily restricted to vibrations around their equilibrium positions. As temperature increases, the amplitude of these vibrations increases, leading to a higher average kinetic energy. This increased vibrational energy can ultimately lead to phase transitions, such as melting.
Liquids: Increased Freedom, Translational and Rotational Motion
In liquids, the particles have more freedom of movement than in solids. They can translate (move from one location to another), rotate, and vibrate. As temperature increases, the particles gain more kinetic energy, leading to increased translational and rotational motion. This increased mobility explains the fluidity of liquids and their tendency to expand with increasing temperature.
Gases: Unrestricted Motion, High Kinetic Energy
Gases exhibit the most energetic motion of the three states. The particles are far apart and experience minimal intermolecular forces. They move freely in all directions, with high translational kinetic energy. As temperature increases, the average kinetic energy of the gas molecules increases significantly, leading to increased pressure and volume if the container is flexible.
Implications and Applications
The relationship between temperature and kinetic energy has profound implications across numerous scientific disciplines and technological applications.
Thermodynamics and Heat Transfer
Understanding this relationship is crucial in thermodynamics, the study of heat and its transformations. Heat transfer is essentially the flow of energy between systems due to temperature differences. This energy flow involves changes in the average kinetic energy of the particles within the systems.
Engine Design and Efficiency
Internal combustion engines rely on the relationship between temperature and kinetic energy to convert heat energy into mechanical work. The combustion of fuel increases the temperature and kinetic energy of the gases in the cylinders, which then exert pressure on the pistons to generate power. Improving engine efficiency often involves optimizing the control and utilization of this kinetic energy.
Material Science and Phase Transitions
The behavior of materials at different temperatures is directly related to the changes in their average kinetic energy. Understanding this relationship is critical in material science, enabling the design of materials with specific properties for various applications. For example, understanding how temperature affects the kinetic energy of polymers allows for the creation of materials with desired flexibility or rigidity.
Chemical Reactions and Reaction Rates
The rate of chemical reactions is often highly temperature-dependent. Higher temperatures mean higher kinetic energy of reactant molecules, leading to more frequent and energetic collisions, thereby increasing the reaction rate. This principle is exploited in numerous industrial processes to accelerate desired chemical reactions.
Meteorology and Climate Science
Temperature and kinetic energy are fundamental to understanding atmospheric phenomena. The movement of air masses, the formation of weather patterns, and the global climate system are all driven by changes in temperature and the resulting kinetic energy of air molecules.
Conclusion: A Fundamental Relationship
The relationship between temperature and kinetic energy is a fundamental concept in physics, underpinning our understanding of matter's behavior and the universe's workings. From the microscopic dance of atoms and molecules to the macroscopic phenomena we observe every day, this relationship provides a framework for comprehending a vast array of natural processes and technological applications. Continued research and exploration of this relationship will undoubtedly lead to further advancements in various fields. Its fundamental nature ensures its enduring significance in our pursuit of knowledge and innovation.
Latest Posts
Latest Posts
-
Solve X 3 1 7 15
Mar 29, 2025
-
How Much Cerebral Capacity Do Dolphins Use
Mar 29, 2025
-
The Original Three Components Of The Cell Theory Are That
Mar 29, 2025
-
Give The Temperature And Pressure At Stp
Mar 29, 2025
-
Give The Ground State Electron Configuration For Pb
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about How Does Temperature Relate To Kinetic Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
