Balanced Equation For Magnesium Metal And Hydrochloric Acid
Juapaving
Mar 10, 2025 · 5 min read
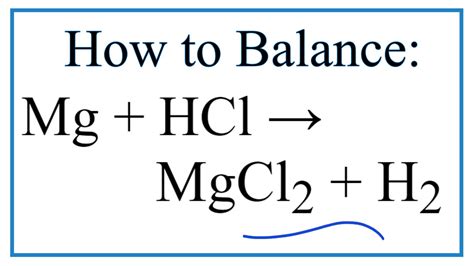
Table of Contents
The Balanced Equation for Magnesium Metal and Hydrochloric Acid: A Deep Dive
The reaction between magnesium metal and hydrochloric acid is a classic example of a single displacement reaction, frequently used in chemistry demonstrations and experiments to illustrate fundamental concepts like stoichiometry, limiting reactants, and gas evolution. Understanding this reaction, including its balanced equation, is crucial for grasping core chemical principles. This comprehensive article will delve into the details of this reaction, exploring its balanced equation, stoichiometry, practical applications, safety considerations, and related concepts.
Understanding the Reaction
Magnesium (Mg), an alkaline earth metal, is highly reactive. Hydrochloric acid (HCl), a strong acid, readily donates protons (H⁺ ions). When magnesium metal is added to hydrochloric acid, a vigorous reaction occurs, producing magnesium chloride (MgCl₂) and hydrogen gas (H₂). This is a single displacement reaction where magnesium displaces hydrogen from the acid.
The Balanced Chemical Equation
The unbalanced equation for the reaction is:
Mg(s) + HCl(aq) → MgCl₂(aq) + H₂(g)
This equation shows the reactants (magnesium and hydrochloric acid) and the products (magnesium chloride and hydrogen gas). However, it's not balanced because the number of atoms of each element isn't equal on both sides of the equation. A balanced chemical equation adheres to the law of conservation of mass, meaning the number of atoms of each element remains constant throughout the reaction.
To balance the equation, we need to adjust the coefficients (the numbers in front of the chemical formulas) to ensure an equal number of atoms on both sides. The balanced equation is:
Mg(s) + 2HCl(aq) → MgCl₂(aq) + H₂(g)
Now, let's verify the balance:
- Magnesium (Mg): 1 atom on both sides.
- Hydrogen (H): 2 atoms on both sides.
- Chlorine (Cl): 2 atoms on both sides.
The equation is now balanced, representing the accurate stoichiometric ratios of reactants and products.
Stoichiometry and Mole Ratios
The balanced equation provides crucial stoichiometric information. The coefficients represent the mole ratios of the reactants and products. In this reaction:
- 1 mole of magnesium reacts with 2 moles of hydrochloric acid.
- This reaction produces 1 mole of magnesium chloride and 1 mole of hydrogen gas.
This information is vital for calculating the amount of reactants needed to produce a specific amount of product or vice-versa. For example, if we want to produce 0.5 moles of hydrogen gas, we would need 0.5 moles of magnesium and 1 mole of hydrochloric acid.
Practical Applications
The reaction between magnesium and hydrochloric acid has several practical applications:
- Hydrogen Gas Production: This reaction is a common laboratory method for generating hydrogen gas. Hydrogen is a valuable fuel and is used in various industrial processes. The purity of the hydrogen gas produced depends on the purity of the reactants.
- Determination of Magnesium Content: By carefully measuring the volume of hydrogen gas produced, we can determine the amount of magnesium present in a sample. This is a common analytical technique used in various fields.
- Educational Demonstrations: The reaction is often used in chemistry classrooms to demonstrate concepts like single displacement reactions, gas evolution, and exothermic reactions (the reaction releases heat).
Safety Precautions
This reaction should always be carried out with appropriate safety precautions:
- Eye Protection: Always wear safety goggles to protect your eyes from splashes of acid or escaping hydrogen gas.
- Gloves: Wear gloves to protect your hands from the corrosive hydrochloric acid.
- Ventilation: The reaction produces hydrogen gas, which is flammable. Ensure adequate ventilation to prevent the accumulation of hydrogen gas and the risk of explosion.
- Acid Handling: Handle hydrochloric acid carefully, avoiding contact with skin or clothing. If accidental contact occurs, immediately flush the affected area with plenty of water.
- Appropriate Disposal: Dispose of the reaction mixture properly according to your institution's safety guidelines.
Related Concepts
Understanding the magnesium and hydrochloric acid reaction helps in understanding several related concepts:
- Single Displacement Reactions: This reaction exemplifies a single displacement (or substitution) reaction, where a more reactive element (magnesium) displaces a less reactive element (hydrogen) from a compound.
- Activity Series: The reactivity of metals is often represented using an activity series. Magnesium is more reactive than hydrogen, allowing it to displace hydrogen from hydrochloric acid.
- Exothermic Reactions: The reaction between magnesium and hydrochloric acid is exothermic, meaning it releases heat. The temperature increase can be significant, depending on the amounts of reactants used.
- Limiting Reactants: If the amounts of magnesium and hydrochloric acid are not in the stoichiometric ratio, one reactant will be completely consumed before the other. This reactant is called the limiting reactant, and it determines the amount of product formed.
- Reaction Rate: The rate of the reaction can be affected by several factors, including the concentration of hydrochloric acid, the surface area of the magnesium metal, and the temperature. A higher concentration of acid, a larger surface area of magnesium (e.g., using magnesium powder instead of a ribbon), and a higher temperature will generally increase the reaction rate.
Further Exploration
The reaction between magnesium and hydrochloric acid provides a fertile ground for further exploration. Students can investigate:
- The effect of different concentrations of HCl on the reaction rate.
- The effect of varying the surface area of the magnesium on the reaction rate.
- Quantitative analysis of hydrogen gas production to determine the stoichiometry of the reaction.
- Comparing the reactivity of different metals with hydrochloric acid.
By understanding the balanced equation and the underlying principles, students can develop a deeper appreciation for the fundamentals of chemistry and its applications.
Conclusion
The balanced equation for the reaction between magnesium metal and hydrochloric acid, Mg(s) + 2HCl(aq) → MgCl₂(aq) + H₂(g), is a cornerstone of introductory chemistry. This reaction provides a practical and readily demonstrable example of fundamental chemical principles such as stoichiometry, limiting reactants, gas evolution, and exothermic reactions. By carefully studying this reaction, and understanding the associated safety precautions, students can gain a solid foundation in chemical concepts and develop their experimental skills. Furthermore, exploring the reaction's variations and applications can inspire further investigations into the fascinating world of chemistry. Remember always to prioritize safety when conducting any chemical experiment.
Latest Posts
Latest Posts
-
Is 20 A Multiple Of 3
Mar 10, 2025
-
Is Water An Element Mixture Or Compound
Mar 10, 2025
-
Can A Heterogeneous Mixture Be Separated
Mar 10, 2025
-
Real Life Example Of Gay Lussacs Law
Mar 10, 2025
-
What Are The Factors Of 77
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about Balanced Equation For Magnesium Metal And Hydrochloric Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
