Is Water An Element Mixture Or Compound
Juapaving
Mar 10, 2025 · 6 min read
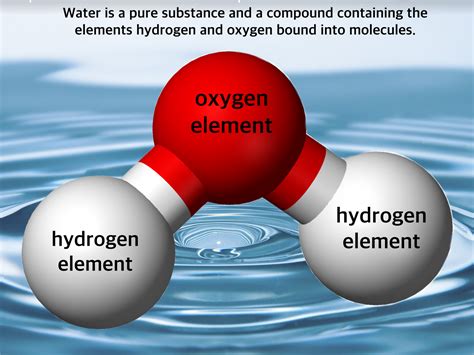
Table of Contents
Is Water an Element, Mixture, or Compound? A Deep Dive into H₂O
The seemingly simple question, "Is water an element, mixture, or compound?" unlocks a fascinating exploration into the fundamental building blocks of matter and the nature of chemical bonding. While the answer might seem straightforward to some, a deeper understanding requires delving into the definitions of elements, mixtures, and compounds, and examining the unique properties of water that arise from its molecular structure. This comprehensive guide will provide a detailed explanation, suitable for students and enthusiasts alike, clarifying the classification of water and highlighting its exceptional characteristics.
Understanding the Basic Definitions
Before we classify water, let's firmly establish the definitions of the key terms: element, mixture, and compound.
Element:
An element is a pure substance consisting only of atoms that all have the same number of protons in their atomic nuclei. This number is known as the atomic number and defines the element. Elements cannot be broken down into simpler substances by chemical means. Examples include oxygen (O), hydrogen (H), iron (Fe), and gold (Au). Elements are the fundamental building blocks of all matter.
Mixture:
A mixture is a substance composed of two or more components not chemically bonded. A key characteristic is that mixtures can be separated into their individual components by physical means, such as filtration, distillation, or evaporation. The composition of a mixture is variable; it can be homogeneous (uniform throughout, like saltwater) or heterogeneous (non-uniform, like sand and water).
Compound:
A compound is a pure substance formed when two or more different elements are chemically bonded together in a fixed ratio. This chemical bonding involves the sharing or transfer of electrons between atoms, creating a new substance with properties different from its constituent elements. Compounds can only be separated into their constituent elements through chemical means. Water (H₂O) is a prime example of a compound.
Water: A Chemical Compound
Based on the definitions above, it's clear that water is a compound. It is formed by the chemical bonding of two elements: hydrogen and oxygen. Specifically, two hydrogen atoms chemically bond with one oxygen atom to form a single molecule of water (H₂O). This bond is a covalent bond, where the hydrogen and oxygen atoms share electrons to achieve a stable electron configuration.
This chemical bonding is crucial. The properties of water are drastically different from the properties of its constituent elements. Hydrogen is a highly flammable gas, while oxygen is a crucial component for combustion. Water, however, is neither flammable nor does it support combustion. This difference exemplifies the fundamental transformation that occurs when elements form a compound.
The Covalent Bond in Water
The covalent bond in water is polar covalent. This means that the electrons are not shared equally between the oxygen and hydrogen atoms. Oxygen is more electronegative than hydrogen, meaning it attracts the shared electrons more strongly. This creates a slightly negative charge (δ-) on the oxygen atom and slightly positive charges (δ+) on the hydrogen atoms. This polarity is responsible for many of water's unique properties.
Unique Properties of Water Resulting from its Compound Nature
The unique properties of water, which are vital for life on Earth, directly stem from its molecular structure and the polar nature of its covalent bonds. Let's explore some key examples:
1. High Specific Heat Capacity:
Water has an exceptionally high specific heat capacity, meaning it takes a significant amount of energy to raise its temperature. This is due to the extensive hydrogen bonding between water molecules. The energy is initially used to break these bonds before increasing the kinetic energy of the molecules and thus raising the temperature. This property helps moderate Earth's climate and maintain stable temperatures in aquatic ecosystems.
2. High Heat of Vaporization:
Similarly, water has a high heat of vaporization, requiring a large amount of energy to convert liquid water into water vapor. This is again due to the strong hydrogen bonds that must be broken during the phase transition. This property makes sweating an effective cooling mechanism for many organisms.
3. Universal Solvent:
Water's polar nature makes it an excellent solvent for many ionic and polar substances. The slightly positive and negative regions of the water molecule can interact with and surround ions and polar molecules, dissolving them. This is crucial for many biological processes, as it allows for the transport of nutrients and other molecules within organisms.
4. Density Anomaly:
Unlike most substances, water is less dense in its solid state (ice) than in its liquid state. This is due to the crystalline structure of ice, which creates more space between water molecules compared to the liquid phase. This property is essential for aquatic life, as ice floats on water, insulating the water below and preventing it from freezing solid.
5. Cohesion and Adhesion:
Water molecules exhibit strong cohesion (attraction to each other) and adhesion (attraction to other substances). Cohesion is due to hydrogen bonding, while adhesion results from the interaction of water molecules with polar surfaces. These properties are critical for processes like capillary action, which allows water to move against gravity in plants.
6. Surface Tension:
Water has a relatively high surface tension due to the strong cohesive forces between its molecules. This property allows for the formation of water droplets and is important for many biological and environmental processes.
Distinguishing Water from Mixtures
It is vital to emphasize the distinction between water and a mixture of hydrogen and oxygen gases. While a mixture of hydrogen and oxygen gases contains the same elements as water, it lacks the crucial characteristic of chemical bonding. A mixture of hydrogen and oxygen gases can be easily separated by physical means (e.g., by diffusion), and it will retain the properties of its individual components. In contrast, water cannot be separated into hydrogen and oxygen by physical means and possesses unique properties distinct from its constituent elements. This distinction is fundamental in differentiating between mixtures and compounds.
Conclusion: Water's Unique Position in the Chemical World
In conclusion, water is unequivocally a compound, not an element or a mixture. Its classification as a compound is rooted in its chemical formation through the covalent bonding of hydrogen and oxygen atoms in a fixed ratio. Moreover, the unique properties of water, essential for life as we know it, are a direct consequence of this chemical bonding and the resulting molecular structure. Understanding water's classification and its remarkable characteristics provides a powerful foundation for comprehending the principles of chemistry and the intricacies of the natural world. The seemingly simple molecule of H₂O is a testament to the complexity and wonder of chemical interactions and their profound influence on our planet. Further exploration into the properties of water and its behavior under various conditions opens up a vast and rewarding field of study.
Latest Posts
Latest Posts
-
What Is An Angle Less Than 90 Degrees
May 12, 2025
-
Name The Two Functional Groups In Amino Acids
May 12, 2025
-
What Is 5 25 In Fraction Form
May 12, 2025
-
Which Part Of The Cell Cycle Takes The Longest
May 12, 2025
-
Which Of These Has Radial Symmetry
May 12, 2025
Related Post
Thank you for visiting our website which covers about Is Water An Element Mixture Or Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.