Can A Heterogeneous Mixture Be Separated
Juapaving
Mar 10, 2025 · 6 min read
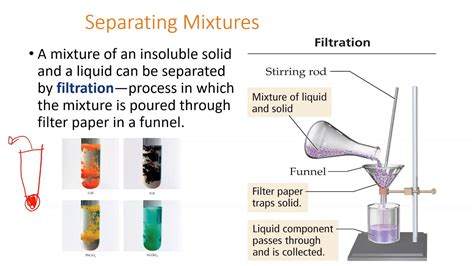
Table of Contents
Can a Heterogeneous Mixture Be Separated? A Comprehensive Guide
Heterogeneous mixtures are everywhere – from the salad on your plate to the rocks in your garden. But what exactly is a heterogeneous mixture, and how do we separate the components that make it up? This comprehensive guide dives deep into the science behind heterogeneous mixtures, exploring the various techniques used to separate their components and providing practical examples to solidify your understanding.
Understanding Heterogeneous Mixtures
A heterogeneous mixture is a type of mixture where the components are not uniformly distributed throughout the mixture. This means you can visually distinguish the different parts of the mixture. Unlike homogeneous mixtures, such as saltwater, where the salt is evenly dissolved and indistinguishable from the water, heterogeneous mixtures show distinct phases or regions. Think of a pizza: you clearly see the cheese, pepperoni, sauce, and crust as separate components.
The key characteristic defining a heterogeneous mixture is the non-uniform distribution of its components. This non-uniformity arises from differences in the physical properties of the constituents, such as size, density, solubility, and magnetic properties. This inherent difference is precisely what allows us to employ various separation techniques.
Examples of Heterogeneous Mixtures
To grasp the concept firmly, let's look at some everyday examples of heterogeneous mixtures:
- Sand and water: The sand particles are clearly visible in the water; they don't dissolve.
- Oil and water: Oil floats on top of the water, forming distinct layers.
- Salad: A mix of various vegetables, each retaining its identity and shape.
- Soil: A complex mixture of minerals, organic matter, water, and air.
- Granite: A rock composed of visible crystals of quartz, feldspar, and mica.
- Concrete: A mixture of cement, sand, gravel, and water.
- Blood: A mixture of cells (red blood cells, white blood cells, platelets) suspended in plasma.
These examples illustrate the diverse nature of heterogeneous mixtures and how easily we can visually identify their individual components.
Separation Techniques for Heterogeneous Mixtures
The separability of a heterogeneous mixture directly stems from the differences in the physical properties of its components. Several techniques exploit these differences for effective separation. Let's examine some of the most common methods:
1. Handpicking: The Simplest Method
This is the most straightforward technique, ideal for separating relatively large and easily distinguishable components. Imagine separating pebbles from a pile of rice: you simply use your hands to pick out the pebbles. Handpicking is effective for separating larger components but becomes impractical for mixtures with many tiny particles.
2. Sieving: Separating by Particle Size
Sieving is a technique used to separate components based on their particle size. A sieve is a mesh with holes of a specific size. By passing the mixture through the sieve, larger particles are retained, while smaller particles pass through. This method is commonly used in separating sand from gravel or separating different sizes of flour.
3. Filtration: Separating Solids from Liquids
Filtration is a fundamental separation technique used to remove solid particles from a liquid. This is achieved by passing the mixture through a filter paper or other porous material. The liquid passes through the filter, while the solid particles are trapped, forming a residue. Coffee filters work on this principle, separating coffee grounds from the brewed coffee. Laboratory filtrations employ specialized filter paper and funnels for more precise separation.
4. Decantation: Separating Liquids of Different Densities
Decantation is a simple process of separating liquids with different densities. It involves carefully pouring the less dense liquid off the top while leaving the denser liquid behind. This is commonly used to separate oil and water, as the oil, being less dense, floats on top. The process requires careful handling to avoid mixing the layers.
5. Evaporation: Separating Dissolved Solids from Liquids
Evaporation is used to separate a dissolved solid from a liquid. By heating the mixture, the liquid evaporates, leaving behind the solid residue. This is frequently employed in salt production from seawater, where the water evaporates, and the salt crystals remain. This method is best suited when the solid is non-volatile, meaning it doesn't evaporate along with the liquid.
6. Sedimentation and Centrifugation: Separating by Density
Sedimentation is a process where denser particles settle at the bottom of a liquid mixture due to gravity. This technique is particularly effective for mixtures with a significant density difference between the components. Centrifugation accelerates this process by using centrifugal force to separate components based on their density. This is extensively used in separating blood components, where blood cells are separated from plasma.
7. Magnetic Separation: Separating Magnetic Materials
This technique exploits the magnetic properties of certain materials. A magnet is used to attract and separate magnetic components from a mixture containing non-magnetic materials. This is often used to separate iron filings from sand or other non-magnetic substances.
8. Distillation: Separating Liquids with Different Boiling Points
Distillation is a more sophisticated technique used to separate liquids with different boiling points. The mixture is heated, and the liquid with the lower boiling point vaporizes first. The vapor is then condensed and collected separately. This method is used in separating alcohol from fermented mixtures or purifying water. Fractional distillation, a more advanced form, is used to separate liquids with boiling points that are closer together.
9. Chromatography: Separating Components based on Adsorption
Chromatography is a powerful separation technique that exploits the differences in the adsorption of different components to a stationary phase. The mixture is passed through a stationary phase (e.g., paper, silica gel), and the components separate based on their differential affinities for the stationary and mobile phases. This is used extensively in analytical chemistry to separate and identify complex mixtures.
10. Sublimation: Separating Solids with Different Sublimation Properties
Sublimation involves the transition of a solid directly to a gas without melting. This method is applicable for separating mixtures where one component sublimes while the other does not. A classic example is separating iodine from a mixture with sand. By heating gently, the iodine will sublime, leaving the sand behind. The iodine vapor can then be condensed to recover the purified iodine.
Choosing the Right Separation Technique
The most appropriate separation technique depends entirely on the properties of the components in the heterogeneous mixture. Consider the following factors:
- Particle size: Sieving is best for mixtures with different particle sizes.
- Density difference: Decantation, sedimentation, and centrifugation are suitable for liquids or suspensions with varying densities.
- Solubility: Filtration and evaporation are useful for separating solids from liquids or dissolved solids from solutions.
- Magnetic properties: Magnetic separation is ideal for separating magnetic materials from non-magnetic ones.
- Boiling points: Distillation is excellent for separating liquids with different boiling points.
- Adsorption properties: Chromatography is crucial for separating complex mixtures based on adsorption differences.
- Sublimation properties: Sublimation is useful for separating solids with different sublimation properties.
Conclusion: The Versatility of Separation Techniques
The ability to separate the components of heterogeneous mixtures is crucial in various fields, from everyday tasks to advanced scientific research. The numerous techniques discussed highlight the versatility and adaptability of separation methods to the unique characteristics of each mixture. Understanding these techniques is essential for anyone working with mixtures, whether in a kitchen, laboratory, or industrial setting. By applying the appropriate technique, we can efficiently separate components and isolate materials of interest, unlocking their individual properties and potential applications. The world of heterogeneous mixtures, while diverse, is beautifully organized and manageable through the power of separation techniques.
Latest Posts
Latest Posts
-
How Big Is 6 Inches In Cm
May 09, 2025
-
How Many Feet In 85 Inches
May 09, 2025
-
Does The Diagonals Of A Parallelogram Bisect Each Other
May 09, 2025
-
Is 88 A Prime Or Composite Number
May 09, 2025
-
Which Of The Following Bonds Is The Weakest
May 09, 2025
Related Post
Thank you for visiting our website which covers about Can A Heterogeneous Mixture Be Separated . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.