Atomic Number Refers To The Number Of In An Atom
Juapaving
Mar 30, 2025 · 6 min read
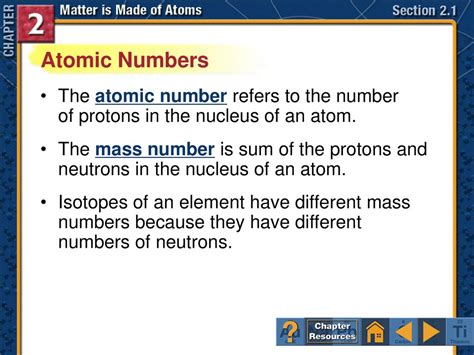
Table of Contents
Atomic Number: Understanding the Identity of an Atom
The atomic number is a fundamental property of an atom, defining its unique identity and chemical behavior. It's a seemingly simple concept, yet it underpins our understanding of the entire periodic table and the interactions between elements. This article will delve deep into the meaning of atomic number, exploring its significance in chemistry, physics, and beyond. We'll also examine how it relates to other atomic properties and its crucial role in determining an element's characteristics.
What is Atomic Number?
The atomic number, denoted by the symbol Z, refers to the number of protons in the nucleus of an atom. This is arguably the single most important characteristic of an element. No two elements have the same atomic number. While atoms of the same element can have varying numbers of neutrons (resulting in isotopes), the number of protons remains constant and defines the element itself.
Protons: The Defining Particle
Protons, along with neutrons, reside in the atom's nucleus – a dense central core. Protons carry a positive electrical charge, while neutrons are electrically neutral. The strong nuclear force overcomes the electrostatic repulsion between the positively charged protons, holding the nucleus together. The atomic number, therefore, directly reflects the positive charge of the nucleus.
Electrons: Balancing the Charge
Atoms are electrically neutral. This means that the number of negatively charged electrons orbiting the nucleus must equal the number of positively charged protons. The arrangement of these electrons in energy levels or shells determines an element's chemical reactivity and the types of bonds it can form. While the number of electrons can change (forming ions), the atomic number remains constant, defining the element's fundamental identity.
The Significance of Atomic Number
The atomic number is not merely a label; it has profound implications for understanding the properties and behavior of elements:
1. Identifying Elements
The most straightforward application of the atomic number is element identification. Each element on the periodic table has a unique atomic number. For instance, hydrogen (H) has an atomic number of 1, helium (He) has 2, lithium (Li) has 3, and so on. This systematic organization is based entirely on the increasing number of protons.
2. Determining Chemical Properties
The arrangement of electrons, determined by the number of protons, directly influences an element's chemical properties. Elements in the same group (vertical column) on the periodic table have similar chemical behaviors due to having the same number of valence electrons – the electrons in the outermost shell. These valence electrons participate in chemical bonding, dictating how an element interacts with other elements.
3. Predicting Periodic Trends
Atomic number allows us to predict trends in various atomic properties across the periodic table. These trends, such as electronegativity (the tendency to attract electrons), ionization energy (the energy required to remove an electron), and atomic radius (the size of an atom), exhibit patterns that are largely explained by the changes in atomic number and electron configuration.
4. Understanding Isotopes
Isotopes are atoms of the same element that have the same atomic number (same number of protons) but differ in their number of neutrons. This difference in neutron number results in variations in atomic mass. For example, carbon-12 and carbon-14 are isotopes of carbon, both having an atomic number of 6, but differing in neutron number (6 and 8, respectively). Understanding isotopes is crucial in various fields, including nuclear medicine and radiocarbon dating.
Atomic Number and the Periodic Table
The periodic table is organized in ascending order of atomic number. This arrangement is not coincidental; it directly reflects the underlying principles governing atomic structure and chemical behavior. The arrangement allows for the prediction and understanding of:
1. Periodic Trends
The periodic table visually represents the periodic trends in atomic properties. As you move across a period (horizontal row), the atomic number increases, leading to predictable changes in atomic radius, ionization energy, and electronegativity. Similarly, moving down a group (vertical column) also leads to predictable trends.
2. Electron Configuration
The atomic number dictates the electron configuration of an element. This configuration, representing the arrangement of electrons in energy levels and sublevels, determines an element's chemical reactivity and bonding behavior. The periodic table's structure helps visualize these electron configurations and their connection to chemical properties.
3. Chemical Reactivity
The periodic table helps predict the chemical reactivity of elements based on their atomic number and electron configuration. Elements in the same group often exhibit similar reactivity due to similar valence electron configurations. For example, the alkali metals (Group 1) are highly reactive because they readily lose one electron to achieve a stable electron configuration.
Atomic Number in Nuclear Physics
Atomic number plays a vital role in nuclear physics, particularly in understanding nuclear reactions and radioactive decay:
1. Nuclear Reactions
In nuclear reactions, the atomic number changes. Nuclear fission (splitting of an atom's nucleus) and nuclear fusion (combining of atomic nuclei) alter the number of protons in the nucleus, resulting in different elements being formed. These changes in atomic number are fundamental to understanding nuclear energy and processes within stars.
2. Radioactive Decay
Radioactive decay involves the spontaneous emission of particles from an unstable nucleus. Different types of radioactive decay can lead to changes in the atomic number of the decaying nucleus. For instance, alpha decay reduces the atomic number by 2, while beta decay increases it by 1. Understanding these changes is critical for nuclear medicine and various applications of radioactive isotopes.
3. Nuclear Stability
The ratio of protons to neutrons in a nucleus determines its stability. Certain combinations of protons and neutrons lead to stable nuclei, while others result in radioactive isotopes. The atomic number is a key factor in predicting the stability of a nucleus and its tendency to undergo radioactive decay.
Applications of Atomic Number
The concept of atomic number has widespread applications in diverse fields:
1. Chemistry
Atomic number is fundamental to all areas of chemistry, from understanding chemical bonding and reactivity to predicting chemical properties and designing new materials. It forms the foundation of chemical nomenclature, allowing for the identification and classification of compounds and elements.
2. Physics
In physics, atomic number is essential for understanding atomic structure, nuclear reactions, and radioactive decay. It's crucial in fields like nuclear physics, astrophysics, and materials science.
3. Medicine
Atomic number plays a vital role in medical applications, particularly in nuclear medicine. Radioactive isotopes, characterized by their atomic number and decay properties, are used for diagnosis and treatment of various diseases.
4. Engineering
Atomic number is used in engineering to understand the properties of materials and design new alloys and materials with specific properties. The selection of materials for various applications often relies on an understanding of their atomic structure and properties, which are dictated by their atomic number.
5. Geology and Archaeology
In geology and archaeology, isotopes with specific atomic numbers are used for radiometric dating, allowing scientists to determine the age of rocks and artifacts. This application relies on understanding the radioactive decay of isotopes and their atomic number.
Conclusion: A Cornerstone of Modern Science
The atomic number, representing the number of protons in an atom, is a cornerstone of modern science. Its significance extends across various disciplines, shaping our understanding of the physical world and enabling technological advancements. From identifying elements and predicting their properties to understanding nuclear reactions and designing new materials, the atomic number remains an indispensable concept, driving progress in chemistry, physics, medicine, engineering, and many other fields. Its simplicity belies its profound importance in shaping our comprehension of the universe at its most fundamental level. The seemingly simple number Z is, in reality, a key that unlocks a vast array of knowledge about the nature of matter itself.
Latest Posts
Latest Posts
-
How Many Rna Polymerases Are Found In Prokaryotes
Apr 01, 2025
-
When Two Parallel Lines Are Crossed By A Transversal
Apr 01, 2025
-
Device That Converts Light Energy Into Electrical Energy
Apr 01, 2025
-
Relationship Between Degree Celsius And Fahrenheit
Apr 01, 2025
-
What Is The Lcm Of 26 And 39
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Atomic Number Refers To The Number Of In An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
