Are Wavelength And Energy Directly Proportional
Juapaving
Mar 22, 2025 · 5 min read
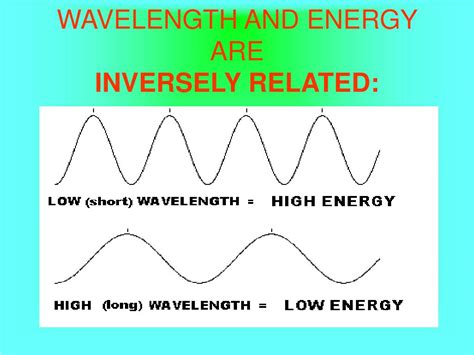
Table of Contents
Are Wavelength and Energy Directly Proportional? An In-Depth Exploration
The relationship between wavelength and energy is a fundamental concept in physics, particularly in the study of light and other electromagnetic waves. A common misconception is that wavelength and energy are directly proportional; however, the relationship is actually inversely proportional. This article delves deep into this relationship, exploring the underlying physics, providing real-world examples, and addressing common misunderstandings.
Understanding Wavelength and Energy
Before diving into the relationship, let's define our key terms:
-
Wavelength (λ): This refers to the distance between two consecutive crests (or troughs) of a wave. It's typically measured in meters (m), nanometers (nm), or other units of length. A longer wavelength signifies a wave that is spread out, while a shorter wavelength indicates a more compressed wave.
-
Energy (E): This represents the capacity to do work. In the context of electromagnetic waves, energy is directly related to the amplitude and frequency of the wave. It's typically measured in Joules (J) or electronvolts (eV). Higher energy waves carry more power and can interact more strongly with matter.
The Inverse Relationship: The Equation and its Implications
The relationship between the wavelength (λ) and energy (E) of a photon (a particle of light) is described by the following equation:
E = hc/λ
Where:
- E is the energy of the photon
- h is Planck's constant (approximately 6.626 x 10^-34 Js)
- c is the speed of light in a vacuum (approximately 3 x 10^8 m/s)
- λ is the wavelength of the photon
This equation clearly demonstrates the inverse proportionality: as the wavelength (λ) increases, the energy (E) decreases, and vice versa. A longer wavelength means the photon has lower energy, while a shorter wavelength means it has higher energy.
Visualizing the Inverse Proportionality
Imagine a wave in the ocean. A long, rolling wave has less energy than a short, choppy wave. The long wave carries its energy over a greater distance, spreading it out, while the short wave packs the same amount of energy into a smaller space, resulting in a more powerful impact. Electromagnetic waves behave similarly.
Real-World Examples of the Inverse Relationship
The inverse relationship between wavelength and energy manifests in numerous ways in the real world:
1. The Electromagnetic Spectrum
The electromagnetic spectrum encompasses all types of electromagnetic radiation, arranged by wavelength (and hence, energy). We can observe the inverse relationship clearly here:
- Radio waves: These have the longest wavelengths and lowest energies.
- Microwaves: Slightly shorter wavelengths and higher energies than radio waves.
- Infrared radiation: Even shorter wavelengths and higher energies than microwaves; felt as heat.
- Visible light: A narrow band of wavelengths and energies we can see; Violet has the shortest wavelength (highest energy), and red has the longest wavelength (lowest energy).
- Ultraviolet (UV) radiation: Shorter wavelengths and higher energies than visible light; can cause sunburn.
- X-rays: Much shorter wavelengths and much higher energies than UV radiation; used in medical imaging.
- Gamma rays: The shortest wavelengths and highest energies; highly penetrating and dangerous.
This progression illustrates how decreasing wavelength directly leads to increasing energy.
2. Photoelectric Effect
The photoelectric effect is a prime example demonstrating the inverse relationship. When light shines on a metal surface, electrons can be emitted. However, this only happens if the light's frequency (and therefore its energy) is above a certain threshold. Light with a longer wavelength (lower energy) won't eject electrons, no matter how intense the light is. This proves that energy, not intensity, is the determining factor in this phenomenon. The shorter the wavelength (higher the energy), the more readily electrons are emitted.
3. Spectroscopy
Spectroscopy is a technique that analyzes the interaction between light and matter. Atoms and molecules absorb and emit light at specific wavelengths, creating unique spectral "fingerprints." The wavelengths of these absorbed or emitted photons reveal information about the energy levels within the atoms or molecules. Higher energy transitions correspond to shorter wavelengths, and lower energy transitions correspond to longer wavelengths.
4. Medical Imaging
Different medical imaging techniques utilize electromagnetic radiation of varying wavelengths and energies. For instance, X-rays, with their short wavelengths and high energies, are capable of penetrating soft tissues to image bones. MRI, on the other hand, uses radio waves with longer wavelengths and lower energies, which interact with the magnetic properties of atomic nuclei to create detailed images of internal organs. The choice of wavelength is crucial because it determines the radiation's penetrating power and interaction with the body's tissues.
Addressing Common Misconceptions
Several misunderstandings surrounding the wavelength-energy relationship are prevalent:
-
Confusing Intensity with Energy: The intensity of light refers to its brightness or power per unit area. While a higher intensity beam contains more photons, the energy of each individual photon is determined solely by its wavelength (and frequency). A high-intensity, low-energy light beam will still have less energetic photons than a low-intensity, high-energy beam.
-
Direct Proportionality with Frequency: While wavelength and energy are inversely proportional, energy and frequency are directly proportional. The relationship is described by the equation: E = hf, where 'f' is the frequency of the wave. A higher frequency means higher energy. Since frequency and wavelength are inversely related (c = fλ), we can see why the energy-wavelength relationship is inverse.
Conclusion: A Crucial Concept in Physics and Beyond
The inverse relationship between wavelength and energy is a cornerstone of physics, with far-reaching implications across various scientific disciplines and technologies. Understanding this relationship is crucial for comprehending phenomena ranging from the behavior of light to the functioning of sophisticated medical imaging equipment. By recognizing that shorter wavelengths equate to higher energy photons and longer wavelengths equate to lower energy photons, we can better understand the world around us at a fundamental level. Remembering the equation E = hc/λ serves as a powerful tool for calculating and interpreting the energy associated with different wavelengths of electromagnetic radiation. This inverse relationship isn't just a theoretical concept; it's a demonstrable reality impacting numerous aspects of our lives.
Latest Posts
Latest Posts
-
Fog Is An Example Of A
Mar 23, 2025
-
What Is The Similarity Between A Compound And A Mixture
Mar 23, 2025
-
How To Spell 18 In Words
Mar 23, 2025
-
Kelvin Planck Second Law Of Thermodynamics
Mar 23, 2025
-
Are There Mitochondria In Plant Cells
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Are Wavelength And Energy Directly Proportional . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
