Write The Electron Configuration For A Neutral Atom Of Germanium
Juapaving
Mar 31, 2025 · 6 min read
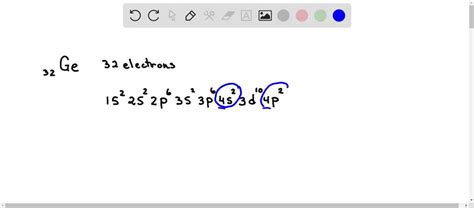
Table of Contents
Unveiling the Electron Configuration of Germanium: A Deep Dive
Germanium, a fascinating metalloid element, holds a unique position in the periodic table, bridging the gap between metals and nonmetals. Understanding its electron configuration is key to comprehending its chemical properties and behavior. This comprehensive guide will not only provide the electron configuration of a neutral germanium atom but also delve into the underlying principles, explore its implications, and offer practical applications.
Understanding Electron Configuration
Before we dive into the specifics of germanium, let's establish a firm understanding of electron configuration. Electron configuration describes the arrangement of electrons within the electron shells and subshells of an atom. This arrangement dictates how an atom interacts with other atoms, forming chemical bonds and determining its chemical properties. It follows the Aufbau principle, which states that electrons fill the lowest energy levels first, and Hund's rule, which dictates that electrons individually occupy orbitals within a subshell before pairing up. The Pauli exclusion principle further dictates that no two electrons within an atom can have the same set of quantum numbers.
Key Concepts: Shells, Subshells, and Orbitals
- Shells: These represent the principal energy levels of an electron, denoted by the principal quantum number (n). As n increases, the energy level increases and the electrons are further from the nucleus.
- Subshells: Within each shell are subshells, designated by the azimuthal quantum number (l), with values of 0, 1, 2, and 3 representing s, p, d, and f subshells respectively. Each subshell can hold a specific number of electrons.
- Orbitals: Orbitals are regions within a subshell where an electron is most likely to be found. Each orbital can hold a maximum of two electrons, with opposite spins.
Determining the Electron Configuration of Germanium (Ge)
Germanium has an atomic number of 32, meaning a neutral atom possesses 32 electrons. To determine its electron configuration, we follow the Aufbau principle and fill the orbitals in order of increasing energy.
The order of filling is generally: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p... and so on.
Following this order, the electron configuration of germanium is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p²
Let's break this down:
- 1s²: The first shell (n=1) contains the 1s subshell, which holds two electrons.
- 2s² 2p⁶: The second shell (n=2) contains the 2s subshell (two electrons) and the 2p subshell (six electrons).
- 3s² 3p⁶: The third shell (n=3) contains the 3s subshell (two electrons) and the 3p subshell (six electrons).
- 4s² 3d¹⁰ 4p²: The fourth shell (n=4) is where things get slightly more complex. The 4s subshell fills first (two electrons), followed by the 3d subshell (ten electrons), and finally the 4p subshell (two electrons).
This configuration reveals that germanium has four valence electrons (the electrons in its outermost shell, the 4th shell). These valence electrons are responsible for germanium's chemical reactivity.
Valence Electrons and Chemical Behavior
The four valence electrons in germanium's outermost shell are crucial in determining its chemical behavior. These electrons are relatively loosely held and can participate in chemical bonding. Germanium exhibits a variety of oxidation states, primarily +2 and +4, reflecting the potential for these valence electrons to be involved in chemical reactions.
Germanium's Chemical Properties: A Consequence of its Electron Configuration
Germanium's position in the periodic table as a metalloid explains its unique chemical properties. It displays characteristics of both metals and nonmetals. While it can form covalent bonds like nonmetals, it also exhibits some metallic properties such as conductivity, although to a lesser extent than true metals. This dual nature is directly related to its electron configuration and the relatively weak attraction of the nucleus to the valence electrons.
Noble Gas Configuration and Shorthand Notation
A more concise way to represent the electron configuration of germanium is using noble gas notation. Noble gases are elements with completely filled electron shells, exhibiting exceptional stability. Argon (Ar) is the noble gas preceding germanium. Its electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶. We can represent germanium's configuration using this as a basis:
[Ar] 4s² 3d¹⁰ 4p²
This shorthand notation simplifies the representation while still conveying all the essential information about the electron arrangement.
Applications of Germanium's Properties
The unique properties of germanium, stemming directly from its electron configuration, lead to a wide range of applications across diverse industries.
Semiconductors and Electronics
Perhaps the most prominent use of germanium lies in its role as a semiconductor. Its ability to conduct electricity under specific conditions, neither as well as a conductor nor as poorly as an insulator, makes it ideal for electronic components such as transistors and diodes. The arrangement of electrons in germanium allows for controlled conductivity, crucial for electronic devices.
Fiber Optics
Germanium dioxide (GeO₂) plays a vital role in the manufacturing of optical fibers. Its high refractive index allows for efficient light transmission through these fibers, making it an essential component in modern telecommunications networks.
Alloys and Catalysts
Germanium alloys are used in various applications, often enhancing the properties of other metals. Its addition can improve the strength, hardness, and corrosion resistance of certain metal alloys. Furthermore, germanium compounds find application as catalysts in several chemical processes.
Infrared Technology
Germanium's ability to transmit infrared radiation makes it indispensable in infrared detectors and optics. These applications range from night vision devices to scientific instruments for studying infrared light sources.
Further Exploration: Excited States and Ions
Our discussion so far has focused on the ground state electron configuration of a neutral germanium atom. However, it's crucial to understand that electrons can absorb energy and jump to higher energy levels, resulting in excited states. These excited states have different electron configurations than the ground state. The subsequent relaxation back to the ground state often involves the emission of photons, the basis for many spectroscopic techniques.
Additionally, germanium can form ions by gaining or losing electrons. For instance, Ge²⁺ and Ge⁴⁺ ions are common, reflecting the potential for the loss of its valence electrons. The electron configurations of these ions will differ from the neutral atom, with fewer electrons in the outermost shell.
Conclusion
The electron configuration of germanium (1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p² or [Ar] 4s² 3d¹⁰ 4p²) is not merely a theoretical concept; it's the cornerstone of understanding the element's chemical behavior and properties. This understanding is essential for appreciating its role in a vast range of technologies and applications, from semiconductors and electronics to fiber optics and infrared technologies. The unique properties of germanium, a consequence of its electron configuration, continue to drive innovation and shape technological advancements across many industries. By delving into the intricacies of its electron arrangement, we gain a deeper appreciation of this remarkable metalloid and its impact on our world.
Latest Posts
Latest Posts
-
What Organelle Is The Site Of Aerobic Respiration
Apr 01, 2025
-
1 5 Gallons Is How Many Liters
Apr 01, 2025
-
How To Turn Gas Into Liquid
Apr 01, 2025
-
What Are The Kinds Of Motion
Apr 01, 2025
-
Describe How You Would Prepare A Supersaturated Solution
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Write The Electron Configuration For A Neutral Atom Of Germanium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
