Describe How You Would Prepare A Supersaturated Solution
Juapaving
Apr 01, 2025 · 6 min read
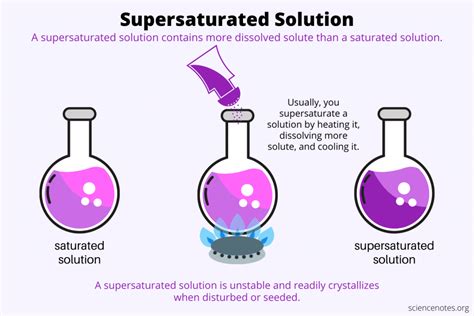
Table of Contents
Preparing a Supersaturated Solution: A Comprehensive Guide
Supersaturated solutions represent a fascinating state of matter, holding more solute than typically possible at a given temperature. Understanding how to prepare these solutions requires a grasp of solubility, equilibrium, and the careful manipulation of these factors. This comprehensive guide will walk you through the process, covering the necessary precautions and offering practical tips for success.
Understanding Solubility and Supersaturation
Before delving into the preparation process, it's crucial to understand the underlying concepts. Solubility refers to the maximum amount of a solute that can dissolve in a specific amount of solvent at a particular temperature and pressure. This is usually expressed in grams of solute per 100 grams of solvent, or as molarity (moles per liter).
A saturated solution contains the maximum amount of solute that can dissolve at equilibrium. Adding more solute to a saturated solution will not result in further dissolution; it will simply remain undissolved at the bottom of the container.
A supersaturated solution, however, contains more solute than its saturation limit. This is a metastable state—meaning it's not thermodynamically stable. The slightest disturbance can cause the excess solute to crystallize out, returning the solution to a saturated state.
Factors Affecting Solubility and Supersaturation
Several factors influence the solubility of a substance and, consequently, the creation of a supersaturated solution. These include:
1. Temperature:
Temperature plays a crucial role. The solubility of most solids in liquids increases with increasing temperature. This means that you can dissolve more solute at higher temperatures. The difference between the amount of solute dissolved at a higher temperature and the amount that can be dissolved at a lower temperature is key to creating a supersaturated solution.
2. Pressure:
Pressure significantly impacts the solubility of gases, but its effect on the solubility of solids is usually negligible. Increasing pressure generally increases the solubility of gases.
3. Nature of Solute and Solvent:
The chemical nature of both the solute and the solvent dictates solubility. "Like dissolves like" is a general rule: polar solvents (like water) tend to dissolve polar solutes, while nonpolar solvents (like hexane) dissolve nonpolar solutes.
4. Presence of Impurities:
Impurities can influence solubility, sometimes increasing it and sometimes decreasing it. This is why using pure solvents and solutes is crucial for reproducible results.
Methods for Preparing a Supersaturated Solution
Several methods can be employed to prepare a supersaturated solution. The success of each method depends on the specific solute and solvent used.
Method 1: Heating and Cooling
This is the most common method. It involves the following steps:
- Heat the solvent: Heat the solvent (e.g., water) to a higher temperature than you intend to work at. The higher the temperature, the more solute you can dissolve.
- Add the solute: Gradually add the solute to the hot solvent, stirring continuously to ensure complete dissolution. Continue adding solute until no more dissolves even with vigorous stirring. You've reached saturation at that temperature.
- Add a little extra: Carefully add a small additional amount of solute. A few extra crystals might dissolve, bringing the solution beyond the saturation point.
- Slow cooling: Allow the solution to cool slowly and undisturbed. This step is critical. Rapid cooling provides nucleation sites for crystallization, preventing supersaturation. Consider using an insulated container to slow the cooling process.
- Protect from disturbance: Once cooled, the solution should be kept undisturbed to maintain its supersaturated state. Even a slight vibration or introduction of a seed crystal (a tiny crystal of the solute) can trigger crystallization.
Example: To prepare a supersaturated solution of sodium acetate in water, you would heat water, add sodium acetate until no more dissolves, add a small amount more, and then allow it to cool slowly and undisturbed.
Method 2: Evaporation
This method relies on reducing the amount of solvent to increase the concentration of solute beyond its saturation point.
- Prepare a saturated solution: Start with a saturated solution of the solute in the solvent at room temperature.
- Gentle evaporation: Gradually evaporate the solvent. This can be done by gently heating the solution or placing it in a well-ventilated area. Avoid rapid evaporation, which can also lead to uncontrolled crystallization.
- Monitor concentration: Closely monitor the solution's concentration. Once you observe the first sign of crystallization, immediately stop the evaporation process.
- Careful handling: Handle the supersaturated solution with care to avoid disturbing it and triggering crystallization.
Method 3: Changing Solvent
This method utilizes the principle of differing solubilities of a solute in different solvents.
- Dissolve in a good solvent: Dissolve the solute in a solvent in which it's highly soluble.
- Add a poor solvent: Gradually add a second solvent in which the solute is less soluble. Do this slowly and carefully, stirring gently.
- Supersaturation: This process can lead to supersaturation if the rate of addition of the poor solvent is controlled precisely. The solute will remain dissolved beyond its normal saturation limit in the mixture of solvents.
- Careful handling: Handle the resulting solution with extreme care to avoid inducing crystallization.
Note: This method is more complex and requires a good understanding of the solubility behavior of the solute in the chosen solvents.
Precautions and Safety Measures
Working with supersaturated solutions requires careful attention to safety and technique:
- Use appropriate protective equipment: Always wear safety goggles and gloves.
- Avoid rapid cooling or agitation: Rapid cooling or sudden agitation can trigger crystallization, potentially causing splattering or spills.
- Handle solutions gently: Avoid jarring or shaking the supersaturated solution to prevent premature crystallization.
- Store in a clean and undisturbed environment: Store the supersaturated solution in a clean, dust-free, and vibration-free environment to maximize its stability.
- Proper disposal: Dispose of chemical solutions according to local regulations and guidelines.
Applications of Supersaturated Solutions
Supersaturated solutions find several applications in various fields:
- Chemical crystal growth: The controlled crystallization from supersaturated solutions is used to grow high-quality single crystals for various applications, such as lasers, electronics, and optics.
- Pharmaceuticals: Supersaturated solutions are employed in drug delivery systems to enhance the solubility and bioavailability of poorly soluble drugs.
- Food science: Supersaturated solutions are used in confectionery to create smoother textures and longer shelf life.
- Chemical engineering: Supersaturated solutions are relevant in various industrial processes involving crystallization, precipitation, and purification.
Troubleshooting Common Issues
Even with careful preparation, you might encounter some difficulties.
- Premature crystallization: If crystallization occurs during the preparation, you might have cooled the solution too quickly or introduced impurities. Try again with slower cooling and ensure the purity of the materials.
- Incomplete dissolution: If the solute doesn't fully dissolve, you might need to increase the temperature or use a more effective solvent.
- Unstable solution: If the solution is unstable and crystallizes easily, you might not have achieved a true supersaturated state. Try repeating the process with more careful control of the temperature and agitation.
Conclusion
Preparing a supersaturated solution is a delicate process that demands patience, precision, and a clear understanding of solubility principles. By following these guidelines and taking the necessary precautions, you can successfully create these remarkable metastable solutions and explore their fascinating properties. Remember that the specific method and conditions will vary depending on the chosen solute and solvent, necessitating experimentation and careful observation. The key to success lies in controlling the rate of cooling and minimizing any disturbances to the solution once it's prepared.
Latest Posts
Latest Posts
-
Flip A Coin Roll A Die
Apr 02, 2025
-
Calculating Ka From A Titration Curve
Apr 02, 2025
-
5 Letter Words Starting With T H I
Apr 02, 2025
-
If 2 Matrix Multiplication Is 0
Apr 02, 2025
-
Find The Square Root Of 121
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Describe How You Would Prepare A Supersaturated Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
