Why Does The Electronegativity Increase Across A Period
Juapaving
Mar 28, 2025 · 6 min read
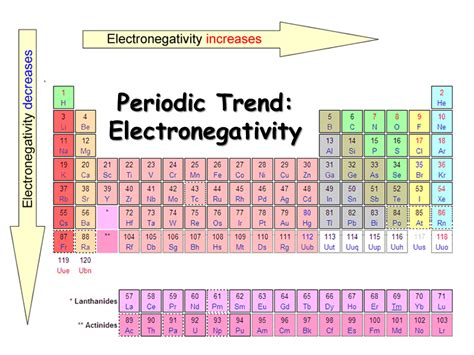
Table of Contents
Why Does Electronegativity Increase Across a Period? A Deep Dive into Atomic Structure and Chemical Bonding
Electronegativity, a fundamental concept in chemistry, dictates the ability of an atom in a molecule to attract shared electrons towards itself. Understanding the trends in electronegativity is crucial for predicting the nature of chemical bonds and the properties of molecules. A consistent observation across the periodic table is the increase in electronegativity across a period (from left to right). But why does this happen? This article delves deep into the atomic structure and the forces at play to explain this crucial trend.
The Atomic Structure: A Foundation for Understanding Electronegativity
To comprehend why electronegativity increases across a period, we need to revisit the fundamental structure of an atom. An atom consists of a positively charged nucleus containing protons and neutrons, surrounded by negatively charged electrons arranged in energy levels or shells. The number of protons in the nucleus determines the atom's atomic number and its identity as a specific element. The electrons in the outermost shell, called valence electrons, are the key players in chemical bonding and, consequently, electronegativity.
Effective Nuclear Charge: The Driving Force
The effective nuclear charge (Z<sub>eff</sub>) is the net positive charge experienced by an electron in a multi-electron atom. It's not simply the total number of protons (the atomic number) because the electrons themselves exert repulsive forces on each other. Inner electrons shield the outer valence electrons from the full positive charge of the nucleus. This shielding effect reduces the effective nuclear charge experienced by the valence electrons.
As we move across a period, the number of protons increases, while the number of inner electrons (shielding electrons) remains relatively constant within the same shell. This leads to a significant increase in the effective nuclear charge. The valence electrons are pulled more strongly towards the nucleus due to this increased positive charge.
Atomic Radius: The Distance Matters
Another crucial factor influencing electronegativity is the atomic radius. Atomic radius refers to the size of an atom. Across a period, the atomic radius generally decreases. This is because, as the effective nuclear charge increases, the valence electrons are drawn closer to the nucleus, resulting in a smaller atomic size.
The decreased atomic radius directly contributes to the increase in electronegativity. The closer the valence electrons are to the nucleus, the stronger the attractive force, making it easier for the atom to attract shared electrons in a chemical bond.
Electronegativity and Chemical Bonding: A Closer Look
Electronegativity plays a crucial role in determining the type of chemical bond formed between atoms. The difference in electronegativity between two atoms determines whether the bond will be covalent (sharing of electrons), ionic (transfer of electrons), or somewhere in between (polar covalent).
Covalent Bonds: Sharing is Caring (but with Electronegativity Differences)
In covalent bonds, atoms share electrons to achieve a stable electron configuration. However, when the atoms involved have different electronegativities, the shared electrons are not shared equally. The atom with the higher electronegativity attracts the shared electrons more strongly, leading to a polar covalent bond. The more electronegative atom acquires a partial negative charge (δ-), while the less electronegative atom acquires a partial positive charge (δ+).
For example, in a water molecule (H₂O), oxygen has a higher electronegativity than hydrogen. The shared electrons are pulled closer to the oxygen atom, giving it a partial negative charge, and leaving the hydrogen atoms with partial positive charges. This unequal sharing of electrons is what makes water a polar molecule, leading to its unique properties.
Ionic Bonds: The Extreme Electronegativity Difference
When the difference in electronegativity between two atoms is very large, one atom essentially "steals" the electrons from the other. This results in the formation of ions: a positively charged cation (the atom that loses electrons) and a negatively charged anion (the atom that gains electrons). The electrostatic attraction between these oppositely charged ions forms an ionic bond.
For example, in sodium chloride (NaCl), chlorine has a much higher electronegativity than sodium. Chlorine effectively pulls an electron away from sodium, forming a Na⁺ cation and a Cl⁻ anion. The strong electrostatic attraction between these ions forms the ionic bond in NaCl.
Factors Affecting Electronegativity Beyond the Period Trend
While the increase in electronegativity across a period is a dominant trend, other factors can influence the electronegativity of an atom. These include:
Electron Shielding: The Inner Electrons' Role
As previously mentioned, inner electrons shield the valence electrons from the full nuclear charge. The effectiveness of this shielding depends on the electron configuration and the orbitals involved. Slight variations in shielding can influence the effective nuclear charge and, consequently, electronegativity.
Penetration Effect: Orbitals Getting Closer
Electrons in different orbitals penetrate the electron cloud differently. Electrons in s orbitals, for example, penetrate closer to the nucleus than electrons in p orbitals. This penetration effect influences the effective nuclear charge felt by the valence electrons and can subtly affect electronegativity.
Exceptions to the General Trend
While the general trend of increasing electronegativity across a period holds true, there can be minor exceptions. These exceptions usually arise from subtle differences in electron shielding, penetration effects, and other factors affecting the effective nuclear charge. These exceptions are often small and do not significantly alter the overall trend.
Applications and Importance of Understanding Electronegativity
Understanding electronegativity trends is paramount in various aspects of chemistry and related fields:
- Predicting the type of chemical bonds: Knowing the electronegativity values of atoms allows us to predict whether a bond will be ionic, covalent, or polar covalent.
- Determining molecular polarity: Molecular polarity, a crucial factor influencing many physical and chemical properties, is directly related to the electronegativity differences within the molecule.
- Understanding chemical reactivity: Electronegativity plays a significant role in determining the reactivity of elements and compounds.
- Designing new materials: In materials science, understanding electronegativity is essential for designing materials with specific properties, such as conductivity or strength.
Conclusion: A Powerful Concept with Wide-Reaching Implications
The increase in electronegativity across a period is a fundamental concept in chemistry driven by the interplay between effective nuclear charge and atomic radius. The increase in effective nuclear charge due to the addition of protons without a corresponding increase in shielding electrons, coupled with the decrease in atomic radius, leads to a stronger attraction of valence electrons to the nucleus. This stronger attraction translates to a higher electronegativity, impacting the nature of chemical bonds formed and the properties of the resulting molecules. Understanding this principle is crucial for comprehending the vast array of chemical phenomena and developing new technologies. While minor exceptions exist, the overall trend remains a cornerstone of chemical understanding.
Latest Posts
Latest Posts
-
54 As Product Of Prime Factors
Mar 31, 2025
-
Organelles That Are The Sites Of Protein Synthesis
Mar 31, 2025
-
What Is The Percentage Of 2 5
Mar 31, 2025
-
How To Find Reciprocal Of A Mixed Number
Mar 31, 2025
-
Which Of The Following Is Considered A Micronutrient
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Why Does The Electronegativity Increase Across A Period . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
