Why Do Atoms Want 8 Valence Electrons
Juapaving
Mar 28, 2025 · 7 min read
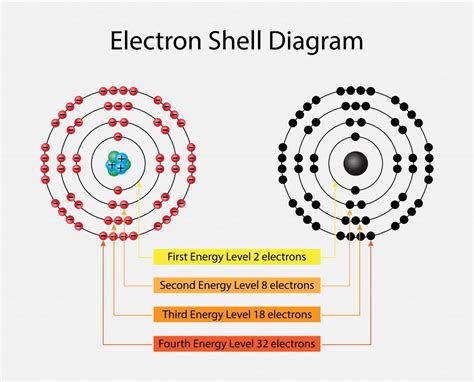
Table of Contents
Why Do Atoms Want 8 Valence Electrons? The Octet Rule Explained
The quest for stability is a driving force in the universe, and it's profoundly reflected in the behavior of atoms. At the heart of chemical bonding lies a fundamental principle: the octet rule. This rule states that atoms tend to gain, lose, or share electrons in order to achieve a full outer shell of eight valence electrons. But why this seemingly magical number eight? This article delves deep into the reasons behind the octet rule, exploring its exceptions and its importance in understanding the structure and properties of matter.
The Significance of Valence Electrons
Before we delve into the "why," let's clarify what we mean by valence electrons. These are the electrons located in the outermost shell, or energy level, of an atom. They're the electrons furthest from the atom's nucleus and, consequently, are the ones most involved in chemical reactions. Think of them as the atom's "social butterflies"—eager to interact with other atoms.
The number of valence electrons an atom possesses determines its chemical reactivity and the types of bonds it can form. Atoms with a nearly full or nearly empty valence shell are particularly reactive, striving to achieve a more stable configuration.
The Noble Gases and the Stable Octet
The key to understanding the octet rule lies in observing the noble gases (Helium, Neon, Argon, Krypton, Xenon, and Radon). These elements are famously inert, meaning they rarely participate in chemical reactions. This remarkable stability stems from their electron configuration: they all have a full outer electron shell.
-
Helium (He), with its atomic number 2, has a full outer shell with two electrons. This is an exception to the octet rule, as it only needs two electrons for a complete outer shell. This is because its outermost shell (the first shell) only has space for two electrons.
-
All other noble gases have eight electrons in their outermost shell, thus fulfilling the octet rule. This complete outer shell provides exceptional stability because it represents a low-energy state. It requires significantly more energy to remove or add electrons to this already complete configuration.
The Energetic Basis of the Octet Rule
The octet rule is not just an empirical observation; it has a strong foundation in quantum mechanics. The stability associated with a filled valence shell arises from the arrangement of electrons in atomic orbitals.
Atomic Orbitals and Electron Configuration
Electrons occupy specific energy levels and sublevels within an atom, described by atomic orbitals (s, p, d, f). The valence electrons reside in the outermost shell, and the s and p orbitals within that shell are particularly crucial for chemical bonding.
- s orbitals can hold a maximum of two electrons.
- p orbitals can hold a maximum of six electrons (three p orbitals, each holding two electrons).
Thus, the outermost shell can accommodate a maximum of eight electrons (two from the s orbital and six from the three p orbitals). A full octet represents a complete filling of these orbitals, resulting in a state of minimal energy and maximum stability.
Electron-Electron Repulsion and Shielding
Another contributing factor to the stability of a filled outer shell is electron-electron repulsion. When more electrons are added to the valence shell, they experience increased repulsion from each other. However, the attractive force of the nucleus counteracts this repulsion. A filled octet represents a balance between these two forces, minimizing repulsion while maximizing attractive forces.
Furthermore, the inner electrons, known as core electrons, shield the valence electrons from the full positive charge of the nucleus. This shielding effect reduces the effective nuclear charge experienced by the valence electrons. A filled octet effectively maximizes this shielding effect, further contributing to stability.
Achieving a Stable Octet: Bonding Mechanisms
Atoms lacking a full octet tend to react with other atoms to achieve this stable configuration. They do so through three primary mechanisms:
1. Ionic Bonding: Electron Transfer
In ionic bonding, one atom transfers one or more electrons to another atom. This results in the formation of ions—charged atoms. For example, in the formation of sodium chloride (NaCl), sodium (Na) loses one electron to become a positively charged ion (Na+), while chlorine (Cl) gains one electron to become a negatively charged ion (Cl-). Both ions then achieve a stable octet. Sodium achieves an octet by losing its single valence electron, leaving a filled outer shell from the previous level. Chlorine achieves an octet by gaining one electron to fill its outermost shell. The electrostatic attraction between these oppositely charged ions forms the ionic bond.
2. Covalent Bonding: Electron Sharing
In covalent bonding, atoms share electrons to achieve a stable octet. This sharing involves the overlapping of atomic orbitals, creating a region of high electron density between the atoms. For instance, in the formation of a water molecule (H₂O), each hydrogen atom shares one electron with the oxygen atom, and oxygen shares two electrons with each hydrogen atom. This results in each hydrogen atom effectively having two electrons (a full outer shell), and oxygen having eight electrons (a full outer shell). The shared electrons form the covalent bonds.
3. Metallic Bonding: Delocalized Electrons
Metallic bonding is characterized by a "sea" of delocalized electrons that are not associated with any particular atom. The valence electrons are shared among all the metal atoms in a crystal lattice structure. This delocalization contributes to the characteristic properties of metals, such as high electrical and thermal conductivity and malleability. While not directly following the octet rule in the same way as ionic and covalent bonding, the sharing of electrons still contributes to overall stability.
Exceptions to the Octet Rule
While the octet rule serves as a powerful guideline in understanding chemical bonding, it's crucial to acknowledge its exceptions. These exceptions arise due to factors such as the availability of d and f orbitals, the size and electronegativity of atoms involved, and the specific energy levels involved.
1. Incomplete Octet:
Some atoms, particularly those of the second period (like boron and beryllium), can form stable compounds with fewer than eight valence electrons. Boron, for example, often forms compounds with only six valence electrons.
2. Expanded Octet:
Atoms in the third period and beyond can have more than eight valence electrons in their compounds, due to the availability of d orbitals. Phosphorus and sulfur are prime examples.
3. Odd-Electron Species:
Some molecules, known as free radicals, possess an odd number of valence electrons, making it impossible for all atoms to achieve an octet. Nitrogen dioxide (NO₂) is a classic example.
The Octet Rule in Predicting Molecular Geometry
The octet rule isn't just crucial for understanding bonding; it also helps predict the geometry of molecules. The arrangement of atoms in a molecule is influenced by the repulsive forces between electron pairs in the valence shell. The VSEPR (Valence Shell Electron Pair Repulsion) theory utilizes the number of electron pairs (both bonding and lone pairs) to predict molecular shapes. This theory is directly linked to the octet rule because it is the desire for atoms to achieve an octet that dictates how many electron pairs will surround the central atom.
Conclusion: A Cornerstone of Chemistry
The octet rule, though possessing exceptions, remains a fundamental principle in chemistry. It offers a simplified yet remarkably effective model for understanding why and how atoms bond, forming the basis for predicting the properties and behavior of molecules. It provides a critical framework for comprehending the diversity and complexity of chemical compounds observed in the world around us. While quantum mechanics provides a deeper understanding of the underlying energetic principles, the octet rule's simplicity makes it an invaluable tool for students and researchers alike. By grasping the principles outlined here, a clearer picture emerges of the elegant dance of electrons that underlies the very fabric of matter.
Latest Posts
Latest Posts
-
Do Isotopes Have The Same Mass Number
Mar 31, 2025
-
Differences Between Renewable And Nonrenewable Resources
Mar 31, 2025
-
Is 16 A Prime Or Composite
Mar 31, 2025
-
Whats The Square Root Of 8
Mar 31, 2025
-
Which Element Is The Most Reactive Metal
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Why Do Atoms Want 8 Valence Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
