Do Isotopes Have The Same Mass Number
Juapaving
Mar 31, 2025 · 5 min read
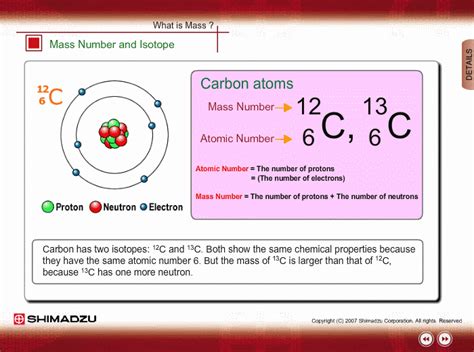
Table of Contents
Do Isotopes Have the Same Mass Number? Understanding Isotopes and Atomic Mass
Isotopes are atoms of the same element that share the same number of protons but differ in the number of neutrons. This fundamental difference leads to variations in their mass numbers, a key concept in understanding atomic structure and nuclear chemistry. This article delves deep into the relationship between isotopes and mass number, clarifying the common misconception that isotopes always have the same mass number. We will explore the definition of isotopes, how mass number is calculated, the implications of isotopic variations, and their significance in various scientific fields.
What are Isotopes?
The identity of an element is primarily determined by its atomic number, which represents the number of protons in its nucleus. All atoms of a particular element possess the same atomic number. However, the number of neutrons in the nucleus can vary, giving rise to isotopes. These are atoms of the same element with the same atomic number but different mass numbers.
For example: Carbon-12 (¹²C), Carbon-13 (¹³C), and Carbon-14 (¹⁴C) are all isotopes of carbon. They all have six protons (atomic number 6), but they differ in their neutron count: six neutrons in ¹²C, seven neutrons in ¹³C, and eight neutrons in ¹⁴C.
Key Characteristics of Isotopes:
- Same atomic number (number of protons): This is the defining characteristic of isotopes. They are variants of the same element.
- Different mass numbers (number of protons + neutrons): The differing neutron count directly impacts the atom's mass.
- Similar chemical properties: Since chemical properties are primarily determined by the electron configuration, which is directly related to the number of protons, isotopes of the same element exhibit similar chemical behaviors.
- Different physical properties: The difference in mass leads to variations in physical properties such as density, melting point, and boiling point. This difference is often subtle but measurable.
- Different nuclear stability: Some isotopes are stable, meaning their nuclei do not spontaneously decay. Others are radioactive, meaning their nuclei are unstable and undergo radioactive decay, emitting particles or energy.
Mass Number: The Sum of Protons and Neutrons
The mass number of an atom is the total number of protons and neutrons in its nucleus. It's represented as a superscript to the left of the element symbol. For example, in ¹⁴C, the mass number is 14. This indicates that the carbon-14 atom has 6 protons + 8 neutrons = 14 nucleons (protons and neutrons).
It's crucial to understand that the mass number is an integer representing the total number of nucleons. It is not the same as the atomic mass (also known as atomic weight), which is a weighted average of the masses of all naturally occurring isotopes of an element, taking into account their relative abundances. Atomic mass is expressed in atomic mass units (amu).
The Misconception: Do all Isotopes have the same Mass Number?
The answer is no. Isotopes, by definition, have different numbers of neutrons, leading to different mass numbers. The only characteristic that isotopes share is their atomic number (number of protons). The mass number directly reflects the number of neutrons, and therefore, different neutron counts result in different mass numbers.
The confusion might stem from the fact that isotopes of an element share similar chemical properties. This similarity can overshadow the fact that they have distinct mass numbers.
The Significance of Isotopic Variations
The existence of isotopes has profound implications across various scientific fields:
1. Radiometric Dating:
Radioactive isotopes, with their known decay rates, are essential tools in radiometric dating. By analyzing the ratio of parent isotopes to daughter isotopes in a sample, scientists can determine the age of geological formations, artifacts, and even the Earth itself. Examples include carbon-14 dating (used to date organic materials) and uranium-lead dating (used to date rocks and minerals).
2. Nuclear Medicine:
Radioactive isotopes are used in nuclear medicine for diagnosis and treatment. For instance, iodine-131 is used in the treatment of thyroid disorders, while technetium-99m is widely used in medical imaging. The specific properties of various radioactive isotopes make them suitable for different applications.
3. Tracer Studies:
Stable and radioactive isotopes are used as tracers in biological and chemical experiments. By incorporating isotopes into molecules, scientists can track their movement and transformation within a system. This technique is crucial in understanding metabolic pathways, chemical reactions, and environmental processes.
4. Nuclear Physics and Chemistry:
The study of isotopes is fundamental to nuclear physics and chemistry. Understanding nuclear reactions, decay processes, and nuclear structure relies heavily on the knowledge of different isotopes and their properties. This understanding has contributed to advancements in nuclear energy and other technological applications.
5. Environmental Science:
Isotopes are used in environmental science to study various processes. For instance, the isotopic composition of water can provide insights into hydrological cycles, while isotopic ratios in atmospheric gases can help understand climate change. These tools aid in understanding complex interactions in ecosystems and their responses to environmental change.
Isotopes and Atomic Mass: A Clarification
While isotopes have different mass numbers, it's important to distinguish this from atomic mass. Atomic mass is the weighted average of the masses of all naturally occurring isotopes of an element, taking into account their relative abundances. This weighted average is often found on the periodic table and represents the average mass of an atom of the element as it exists in nature.
The fact that atomic mass is not a whole number reflects the existence of multiple isotopes and their varying abundances. It represents the average mass, not the mass of any single isotope.
Conclusion: Understanding the Nuances of Isotopes and Mass Number
Isotopes, while sharing the same atomic number, have different mass numbers due to variations in their neutron counts. This fundamental difference has profound implications across various scientific fields. Understanding the distinction between isotopes, mass number, and atomic mass is crucial for comprehending atomic structure, nuclear chemistry, and the diverse applications of isotopic analysis. The misconception that all isotopes share the same mass number stems from a lack of clarity regarding the definition of isotopes and the impact of neutron variation. By clarifying these concepts, we can appreciate the crucial role isotopes play in scientific advancements and our understanding of the natural world. Further research into isotopic analysis continues to reveal intricate details about the composition of matter and the processes that shape our planet and universe.
Latest Posts
Latest Posts
-
Sum Of Exterior Angles Of A Quadrilateral
Apr 01, 2025
-
When Heating A Liquid In A Test Tube You Should
Apr 01, 2025
-
How Many Bones Do Shark Have
Apr 01, 2025
-
Least Common Multiple 12 And 18
Apr 01, 2025
-
What Phase Is The Reverse Of Prophase
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Do Isotopes Have The Same Mass Number . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
