Why Are Most Ionic Substances Brittle
Juapaving
Mar 27, 2025 · 6 min read
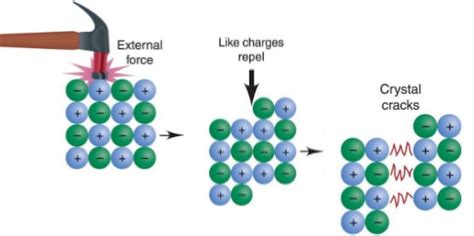
Table of Contents
Why Are Most Ionic Substances Brittle?
Ionic compounds, characterized by the strong electrostatic attraction between oppositely charged ions, exhibit a fascinating property: brittleness. Unlike many metals which can deform under stress, ionic solids shatter easily upon impact. Understanding this inherent fragility requires delving into the crystal structure and the nature of ionic bonds. This article will explore the microscopic reasons behind the brittleness of ionic substances, examining the role of electrostatic forces, crystal lattice structure, and the impact of applied stress.
The Strong Yet Delicate Dance of Ions
The defining characteristic of ionic compounds is the presence of strong electrostatic forces between positively charged cations and negatively charged anions. These forces are significantly stronger than the intermolecular forces found in covalent molecular substances. This strength is responsible for the high melting and boiling points typically observed in ionic compounds. However, this same strength contributes significantly to their brittleness.
The Crystal Lattice: A Highly Ordered Arrangement
Ionic compounds typically exist as crystalline solids. This means the ions are arranged in a highly ordered, three-dimensional lattice structure. The arrangement is dictated by the size and charge of the ions involved, aiming to maximize electrostatic attraction and minimize repulsion. Common structures include cubic close-packed (ccp), hexagonal close-packed (hcp), and body-centered cubic (bcc) arrangements, all optimized for efficient packing and charge balance. This structured arrangement is crucial to understanding why ionic compounds are brittle.
Electrostatic Forces: The Glue and the Weakness
The strong electrostatic attractions between the ions are the “glue” holding the crystal lattice together. However, this very strength becomes a source of weakness when external stress is applied. This is where the delicate balance of attractive and repulsive forces comes into play. The strength of the electrostatic attraction is directly proportional to the charges of the ions and inversely proportional to the square of the distance between them (Coulomb's Law). Any disruption to this delicate balance can lead to catastrophic failure.
What Happens When Stress is Applied?
When an external force is applied to an ionic crystal, the ions are displaced from their equilibrium positions. Imagine pushing on one layer of ions in the lattice. This causes the cations to come closer to other cations and anions to come closer to other anions.
The Role of Repulsion
The key point here is that like charges repel. When the ions are forced into positions where like charges are closer together, the repulsive forces between them rapidly overcome the attractive forces between unlike charges. This repulsion is significantly stronger than the attractive force between the ions in their original positions and can cause the crystal lattice to fracture.
Shear Stress and Cleavage Planes
The application of shear stress – a force that causes layers of the crystal to slide past each other – is particularly damaging to ionic crystals. This sliding motion brings ions of the same charge into close proximity, leading to intense repulsive forces and causing the crystal to cleave along specific planes. These planes, known as cleavage planes, are characterized by a high density of ions of the same charge, minimizing the energy required for fracture. The crystal effectively separates along these planes of weakness, resulting in a clean break.
The Lack of Mobile Charge Carriers
Unlike metallic solids, ionic compounds lack a sea of delocalized electrons. This absence of mobile charge carriers prevents the material from easily deforming under stress. In metals, the electron sea allows the metal ions to slide past each other without significant disruption to the overall structure. This contributes significantly to the malleability and ductility of metals. Ionic solids, lacking this electron sea, have no such mechanism to accommodate deformation.
Comparing Ionic Compounds with Metals and Covalent Networks
To further understand the brittleness of ionic substances, it's helpful to compare them to other types of materials:
Metals: A Sea of Electrons Enables Ductility
Metals possess a "sea" of delocalized electrons that surround positively charged metal ions. When a force is applied, these electrons allow the metal ions to shift past one another without encountering strong repulsive forces. This accounts for the malleability and ductility of metals.
Covalent Network Solids: Strong Bonds, Variable Brittleness
Covalent network solids, such as diamond and silicon carbide, are characterized by strong covalent bonds extending throughout the entire structure. Their brittleness can vary. While many are brittle, some possess a degree of hardness and strength, which is due to the uniform, strong, and directional bonding. Fracture occurs when these strong bonds break, leading to a catastrophic failure. However, the fracture mechanism differs from that of ionic solids.
Factors Influencing Brittleness
Several factors influence the degree of brittleness observed in ionic substances:
Ionic Radius and Charge
The size and charge of the ions significantly impact the strength of the electrostatic forces and the overall stability of the crystal lattice. Larger ions generally result in a weaker electrostatic attraction, while higher charges lead to stronger attraction. The balance between these two factors influences the compound's susceptibility to fracture.
Crystal Structure
The specific arrangement of ions in the crystal lattice also affects brittleness. Certain crystal structures may be more prone to cleavage along specific planes, leading to increased brittleness.
Temperature
At higher temperatures, the increased kinetic energy of the ions can help to absorb some of the energy from an applied stress, making the material slightly less brittle. However, this effect is generally limited. As temperature increases, the thermal vibrations of the lattice are stronger and increase the possibility that the repulsive forces overwhelm the attractive forces.
Impurities and Defects
The presence of impurities or defects in the crystal lattice can disrupt the ordered arrangement of ions, creating points of weakness and increasing the material's susceptibility to fracture.
Applications and Implications
The brittleness of ionic compounds is both a limitation and an asset depending on the application. The fragility can be a drawback in engineering applications where strength and flexibility are crucial. However, this property can also be exploited. The ease with which ionic compounds fracture can be advantageous in applications such as grinding and polishing. Furthermore, certain ionic substances exhibit piezoelectric properties, converting mechanical pressure into electrical energy, a property directly related to their crystal structure and the response to stress.
Conclusion
The brittleness of ionic substances is a direct consequence of the strong electrostatic forces that bind the ions together in a rigid crystal lattice. The inability of ions to slide past each other under stress, combined with the strong repulsive forces between like charges when subjected to shear stress, leads to cleavage and fracture along specific crystallographic planes. Understanding the interplay between electrostatic forces, crystal structure, and applied stress is critical for predicting and controlling the behavior of ionic materials. While their brittleness might pose limitations in certain areas, it also represents a unique property exploited in various applications, highlighting the multifaceted nature of these materials. The study of ionic compounds and their properties continues to be an active area of research with implications for materials science, engineering, and numerous other disciplines.
Latest Posts
Latest Posts
-
There Is A Repulsive Force Between Two Charged Objects When
Mar 30, 2025
-
What Is The Relationship Between The Following Compounds
Mar 30, 2025
-
5 Letter Words Starting With A P
Mar 30, 2025
-
How Many Hundreds Are In 7000
Mar 30, 2025
-
Write 72 As A Product Of Prime Factors
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Why Are Most Ionic Substances Brittle . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
