What Is The Relationship Between The Following Compounds
Juapaving
Mar 30, 2025 · 6 min read
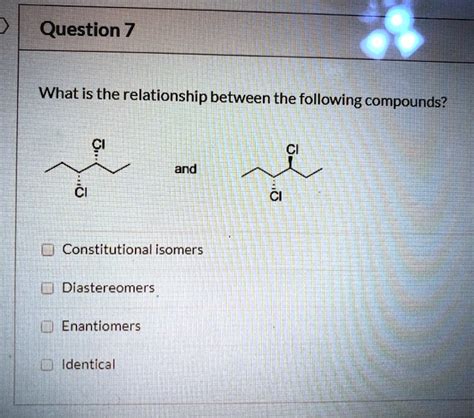
Table of Contents
Unveiling the Intertwined World of Chemical Compounds: Exploring Relationships Between Diverse Molecules
The world of chemistry is a vast and intricate network of interconnected compounds, each with unique properties and behaviors. Understanding the relationships between different molecules is crucial for comprehending a wide range of phenomena, from biological processes to industrial applications. This article delves into the intricate relationships between various chemical compounds, focusing on their structural similarities, functional group interactions, and chemical transformations. We will explore how these relationships dictate their properties and how they interact within various systems.
I. Defining Chemical Relationships:
Before delving into specific examples, let's establish a framework for understanding the types of relationships that can exist between chemical compounds:
-
Structural Isomerism: Compounds with the same molecular formula but different structural arrangements are called structural isomers. This difference can be in the connectivity of atoms (chain isomerism, positional isomerism), the position of functional groups (functional group isomerism), or the presence of a ring structure (ring-chain isomerism). The properties of structural isomers can vary significantly despite sharing the same molecular formula.
-
Stereoisomerism: Stereoisomers possess the same molecular formula and the same connectivity of atoms but differ in the spatial arrangement of atoms in 3D space. This includes geometric isomerism (cis-trans or E-Z isomerism) and optical isomerism (enantiomers and diastereomers). Stereoisomers often exhibit subtle yet critical differences in their biological activity and physical properties.
-
Functional Group Relationships: Compounds containing the same functional group often share similar chemical properties and reactivity. For example, alcohols (-OH group), carboxylic acids (-COOH group), and ketones (C=O group) display characteristic reactions due to the presence of their respective functional groups. Understanding functional group relationships is fundamental to predicting chemical behavior.
-
Homologous Series: In organic chemistry, a homologous series is a group of compounds that differ by a constant unit, usually a CH₂ group. Alkanes, alkenes, and alkynes are classic examples of homologous series. Members of the same series exhibit gradual changes in properties with increasing chain length.
-
Derivatives: One compound can be considered a derivative of another if it's formed through a chemical modification of the parent compound. For instance, esters are derivatives of carboxylic acids, formed through esterification reactions.
II. Exploring Specific Compound Relationships:
Let's now examine specific examples to illustrate these relationships in more detail. For the sake of this discussion, we'll need to select some specific compound classes to analyze. To make it concrete, let's consider the relationships between:
-
Alkanes, Alkenes, and Alkynes: These hydrocarbons represent a classic homologous series. Alkanes (saturated) contain only single bonds, alkenes (unsaturated) have at least one carbon-carbon double bond, and alkynes (unsaturated) contain at least one carbon-carbon triple bond. Their reactivity differs significantly, with alkenes and alkynes being far more reactive due to the presence of pi bonds. This reactivity difference stems directly from the differing bond orders and electron distribution.
-
Alcohols and Ethers: Both alcohols (R-OH) and ethers (R-O-R) contain oxygen atoms, but the connectivity differs. Alcohols have a hydroxyl group (-OH) bonded to a carbon atom, while ethers have an oxygen atom bonded to two carbon atoms. This difference leads to distinct chemical properties. Alcohols undergo oxidation reactions more readily than ethers, and their hydrogen bonding capacity results in higher boiling points.
-
Carboxylic Acids, Esters, and Amides: This group exemplifies derivative relationships. Carboxylic acids (R-COOH) are the parent compounds. Esters (R-COO-R') are formed by the reaction of a carboxylic acid with an alcohol, while amides (R-CONH₂) are formed by the reaction of a carboxylic acid with an amine. The properties of esters and amides differ from carboxylic acids due to the altered functional groups, influencing their polarity, reactivity, and boiling points.
-
Aromatic Compounds and their Derivatives: Benzene (C₆H₆) is a quintessential aromatic compound characterized by its delocalized pi electron system. Numerous derivatives of benzene are formed by substituting one or more hydrogen atoms with various functional groups. These derivatives, such as phenol (benzene with a hydroxyl group), toluene (benzene with a methyl group), and benzoic acid (benzene with a carboxyl group), exhibit properties that reflect both the aromatic ring and the specific substituents. The electron-donating or electron-withdrawing nature of the substituent can significantly influence the reactivity of the benzene ring.
III. Chemical Transformations and Relationships:
Chemical transformations play a vital role in establishing relationships between compounds. Many compounds are synthesized from simpler precursors through well-defined reaction pathways. Understanding these transformations allows us to predict the properties of the products and to design synthetic routes to produce desired molecules. For example:
-
Oxidation and Reduction: These are fundamental redox reactions that interconvert many organic functional groups. Alcohols can be oxidized to aldehydes or ketones, while aldehydes can be further oxidized to carboxylic acids. Conversely, carboxylic acids can be reduced to alcohols.
-
Hydrolysis Reactions: Hydrolysis involves the cleavage of a bond by the addition of water. Esters are hydrolyzed to carboxylic acids and alcohols, while amides are hydrolyzed to carboxylic acids and amines.
-
Substitution Reactions: In substitution reactions, an atom or a group of atoms is replaced by another atom or group. Aromatic compounds undergo substitution reactions where a hydrogen atom is replaced by another functional group, leading to the formation of various derivatives.
-
Addition Reactions: Unsaturated compounds, like alkenes and alkynes, undergo addition reactions, where atoms are added across the multiple bonds. This process saturates the multiple bonds, leading to the formation of new compounds.
IV. Significance of Understanding Compound Relationships:
Understanding the relationships between chemical compounds has far-reaching implications in various fields:
-
Drug Discovery and Development: Identifying structural similarities and differences between molecules is crucial for designing new drugs and predicting their biological activity. Understanding stereoisomerism is paramount, as even minor differences in spatial arrangement can drastically affect the interaction with biological targets.
-
Materials Science: The properties of materials are largely determined by the chemical composition and interactions between the constituent molecules. Understanding the relationships between different polymers, for example, allows for the design of materials with specific properties, such as strength, flexibility, and thermal stability.
-
Environmental Chemistry: Understanding the relationships between pollutants and their degradation products is essential for environmental remediation efforts. Knowing how pollutants transform and interact in the environment helps in developing effective strategies for pollution control.
-
Industrial Chemistry: Many industrial processes involve the transformation of one compound into another. Understanding the relationships between reactants and products is crucial for optimizing reaction conditions and maximizing yield.
V. Conclusion:
The intricate relationships between chemical compounds form the bedrock of chemical science. Exploring structural similarities, functional group interactions, and chemical transformations provides a powerful framework for understanding the properties and behaviors of molecules. This knowledge is fundamental to advancements in various fields, from medicine and materials science to environmental protection and industrial processes. Continued research into the relationships between chemical compounds will undoubtedly unveil even more profound insights into the complexities of the chemical world and unlock new possibilities for innovation and technological advancement. Further exploration of specific chemical families, reaction mechanisms, and advanced spectroscopic techniques will continue to deepen our understanding of these interconnections. The study of chemical relationships is a continuous journey of discovery, revealing the remarkable interconnectedness of matter and the elegance of chemical principles.
Latest Posts
Latest Posts
-
How Many Bones Do Shark Have
Apr 01, 2025
-
Least Common Multiple 12 And 18
Apr 01, 2025
-
What Phase Is The Reverse Of Prophase
Apr 01, 2025
-
Is Tungsten A Metal Or Nonmetal
Apr 01, 2025
-
How Many Seconds Is 24 Hours
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Relationship Between The Following Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
