Why Are Atoms Neutral Despite Having Charged Particles
Juapaving
Mar 31, 2025 · 6 min read
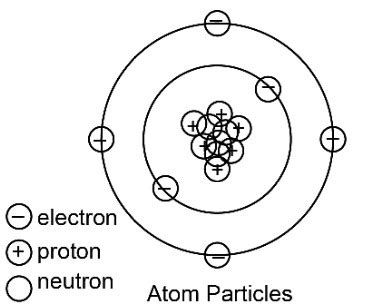
Table of Contents
Why Are Atoms Neutral Despite Having Charged Particles?
The seemingly paradoxical nature of atoms—composed of charged particles yet electrically neutral—is a fundamental concept in chemistry and physics. Understanding this neutrality requires delving into the intricate structure of the atom and the precise balance of its constituent components. This article explores this fascinating topic, explaining the reasons behind atomic neutrality and examining the exceptions to this rule.
The Structure of an Atom: A Balancing Act
Atoms are the fundamental building blocks of matter, comprising three primary subatomic particles:
- Protons: Positively charged particles residing in the atom's nucleus.
- Neutrons: Neutrally charged particles also found within the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels.
The key to understanding atomic neutrality lies in the equal number of protons and electrons present in a neutral atom. The positive charge of each proton precisely cancels out the negative charge of each electron. Neutrons, possessing no charge, don't contribute to the overall charge of the atom. This delicate balance results in a net charge of zero, making the atom electrically neutral.
The Nucleus: A Dense Core of Charge
The atom's nucleus, a tiny region at its center, houses both protons and neutrons. Protons, being positively charged, exert strong repulsive forces on each other. However, this repulsive force is overcome by the strong nuclear force, an incredibly powerful fundamental force that binds protons and neutrons together within the nucleus. This strong force is crucial for the stability of atoms, especially those with larger nuclei containing many protons.
Electron Shells: Orbiting Clouds of Negativity
Electrons, significantly lighter than protons and neutrons, occupy the space surrounding the nucleus in distinct energy levels called electron shells or orbitals. These shells are not fixed pathways, but rather regions of probability where electrons are most likely to be found. The electrons in the outermost shell, known as valence electrons, are particularly important in determining an atom's chemical behavior and reactivity. The number of valence electrons directly influences how an atom will interact with other atoms to form chemical bonds.
Coulomb's Law and the Attraction Between Protons and Electrons
The force of attraction between the positively charged protons in the nucleus and the negatively charged electrons orbiting it is governed by Coulomb's Law. This law states that the electrostatic force between two charged particles is directly proportional to the product of their charges and inversely proportional to the square of the distance between them. In simpler terms, the stronger the charges and the closer they are, the greater the attractive force. This attractive force is what keeps the electrons bound to the nucleus, preventing them from escaping.
The Significance of Distance and Shielding
The distance between the nucleus and the electrons plays a crucial role in determining the strength of the electrostatic attraction. Electrons in inner shells are closer to the nucleus and experience a stronger attractive force than electrons in outer shells. Furthermore, inner shell electrons partially shield outer shell electrons from the full positive charge of the nucleus, reducing the effective nuclear charge experienced by the outer electrons. This shielding effect influences the chemical properties of atoms.
Ions: Exceptions to the Rule of Neutrality
While most atoms exist in a neutral state, it's important to acknowledge the existence of ions. Ions are atoms or molecules that have gained or lost electrons, resulting in a net positive or negative charge.
-
Cations: Positively charged ions formed when an atom loses one or more electrons. The loss of negatively charged electrons leaves the atom with a surplus of positive charge from the protons.
-
Anions: Negatively charged ions formed when an atom gains one or more electrons. The addition of negatively charged electrons results in an excess of negative charge.
The formation of ions is a fundamental process in many chemical reactions and plays a crucial role in the behavior of many materials. Ionic compounds, for example, are formed by the electrostatic attraction between cations and anions.
Ionization Energy and Electron Affinity
The energy required to remove an electron from a neutral atom is known as its ionization energy. Atoms with low ionization energies readily lose electrons to form cations. Conversely, the energy released when an atom gains an electron is known as its electron affinity. Atoms with high electron affinities readily gain electrons to form anions. These properties are influenced by factors like atomic size, nuclear charge, and electron shielding.
Isotopes: Variations in Neutron Number
Isotopes are atoms of the same element that have the same number of protons but differ in the number of neutrons. While the number of neutrons affects the atom's mass, it doesn't change its charge. This is because neutrons are electrically neutral. Therefore, isotopes of the same element retain the same atomic number (number of protons) and are still electrically neutral.
Beyond the Basics: A Deeper Dive into Atomic Structure
The model of the atom presented here is a simplified representation. A more accurate description involves concepts like quantum mechanics, which explains the behavior of electrons in terms of probability distributions and wave functions. This more complex model accounts for the intricacies of electron orbitals and the various energy levels within an atom.
Quantum Mechanics and Atomic Orbitals
Quantum mechanics provides a sophisticated framework for understanding electron behavior. It explains that electrons don't orbit the nucleus in well-defined paths, but rather exist in a cloud of probability described by atomic orbitals. These orbitals have specific shapes and energy levels, determined by quantum numbers. Understanding these quantum numbers is crucial for comprehending electron configuration and the chemical properties of atoms.
Electron Configuration and the Periodic Table
The arrangement of electrons in an atom's orbitals is called its electron configuration. This configuration determines an atom's chemical reactivity and influences its position on the periodic table. The periodic table is organized based on the periodic recurrence of similar electron configurations and chemical properties.
Nuclear Physics and Radioactive Decay
The study of atomic nuclei and their transformations is known as nuclear physics. This field encompasses phenomena like radioactive decay, where unstable nuclei emit particles or energy to achieve a more stable configuration. Radioactive decay can result in changes in the number of protons and neutrons in the nucleus, potentially affecting the atom's charge and stability.
Conclusion: A Delicate Balance of Charges
The neutrality of atoms, despite containing charged particles, stems from the precise balance between the number of protons and electrons. This balance is maintained by the strong electrostatic attraction between these particles, governed by Coulomb's Law. While ions represent exceptions to this rule, their formation involves the gain or loss of electrons, thereby altering the overall charge. Understanding the structure and behavior of atoms, including the concepts of isotopes and the more sophisticated models offered by quantum mechanics, is crucial for comprehending the fundamental principles of chemistry and physics. The intricate interplay of forces within an atom, ultimately leading to its neutrality, highlights the remarkable elegance and complexity of the natural world.
Latest Posts
Latest Posts
-
Round 45 To The Nearest 10
Apr 02, 2025
-
What Is The Length Of A Line Segment
Apr 02, 2025
-
What Is The Ocean Between North America And Europe
Apr 02, 2025
-
How Are Compounds And Mixtures Alike
Apr 02, 2025
-
A Man Stands 10 M In Front
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Why Are Atoms Neutral Despite Having Charged Particles . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
