Which Two Functional Groups Are Always Found In Amino Acids
Juapaving
Mar 19, 2025 · 7 min read
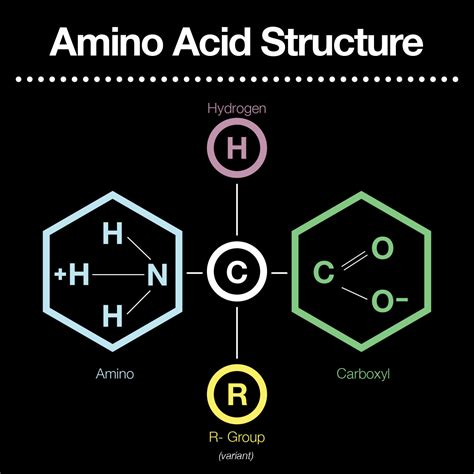
Table of Contents
Which Two Functional Groups Are Always Found in Amino Acids?
Amino acids are the fundamental building blocks of proteins, the workhorses of life. Their remarkable versatility and ability to form complex structures stem from their unique chemical composition, specifically the presence of two crucial functional groups: the amino group (-NH2) and the carboxyl group (-COOH). Understanding these functional groups and their properties is key to grasping the intricacies of protein structure, function, and biological significance. This article delves deep into the characteristics of these groups, their roles in amino acid properties, and the implications for protein formation and biological processes.
The Ubiquitous Amino Group (-NH2)
The amino group, a defining feature of amino acids, is a nitrogen-containing functional group consisting of one nitrogen atom covalently bonded to two hydrogen atoms. This group's presence imparts several critical properties to amino acids:
1. Basic Properties and Protonation:
The nitrogen atom in the amino group possesses a lone pair of electrons, making it a weak base. This means it can readily accept a proton (H+) from a solution, becoming positively charged and forming an ammonium ion (-NH3+). This protonation behavior is highly pH-dependent; at physiological pH (around 7.4), the amino group exists predominantly as an ammonium ion. This ability to accept a proton contributes significantly to the buffering capacity of amino acids and proteins, helping to maintain a stable pH environment within cells.
2. Hydrogen Bonding Capabilities:
The amino group’s nitrogen and hydrogen atoms participate in hydrogen bonding, a crucial intermolecular force responsible for the stability and three-dimensional structure of proteins. The partial positive charge on the hydrogen atoms and the partial negative charge on the nitrogen atom allow for the formation of hydrogen bonds with other molecules, including water, other amino acids, and the peptide backbone itself. These interactions are fundamental for maintaining the secondary, tertiary, and quaternary structures of proteins.
3. Role in Peptide Bond Formation:
The amino group plays a critical role in the formation of peptide bonds, the covalent links that connect amino acids to form polypeptide chains. During peptide bond formation, the carboxyl group of one amino acid reacts with the amino group of another amino acid, releasing a molecule of water and forming a peptide bond (-CONH-). This reaction is a condensation reaction, and the resulting peptide bond is a strong covalent bond that holds the amino acid sequence together.
4. Chemical Modification and Diversity:
The amino group can undergo various chemical modifications, which contribute to the diversity and functionality of amino acids and proteins. These modifications can alter the properties of the amino acid, influencing its interactions with other molecules and its role in protein function. For example, some amino acids have their amino groups methylated or acetylated, impacting protein stability and regulation.
The Essential Carboxyl Group (-COOH)
The carboxyl group, another indispensable functional group, consists of a carbon atom double-bonded to an oxygen atom and singly bonded to a hydroxyl group (-OH). This group bestows several critical characteristics onto amino acids:
1. Acidic Properties and Deprotonation:
The carboxyl group is a weak acid, meaning it can donate a proton (H+) to a solution. The hydroxyl group's oxygen-hydrogen bond is relatively weak, and the resulting carboxylate ion (-COO-) is resonance-stabilized, making this proton donation favorable. At physiological pH, the carboxyl group exists primarily as the deprotonated carboxylate ion, carrying a negative charge. This negative charge contributes significantly to the overall charge of the amino acid and influences its interactions with other molecules.
2. Peptide Bond Formation:
As already mentioned, the carboxyl group is essential for peptide bond formation. It provides the carbonyl carbon atom that participates in the nucleophilic attack by the amino group of another amino acid, forming the amide linkage that constitutes the peptide bond. The release of water during this condensation reaction is crucial for the creation of the polypeptide chain.
3. Chemical Reactivity and Modification:
The carboxyl group exhibits significant chemical reactivity, participating in various reactions, including esterification, amidation, and decarboxylation. These reactions are important in many metabolic processes and post-translational modifications of proteins. Esterification, for example, can modify proteins by adding fatty acyl groups, altering their interactions with membranes. Decarboxylation reactions remove the carboxyl group, often yielding amines and carbon dioxide.
4. Influence on Amino Acid pKa:
The pKa value of the carboxyl group is a measure of its acidity. It influences the charge and properties of the amino acid at different pH values. The pKa of the carboxyl group is typically around 2.0. This means that at a pH below 2.0, the carboxyl group will be largely protonated (neutral), while at pH values above 2.0, it will be mostly deprotonated (negatively charged).
The Zwitterionic Nature of Amino Acids
Due to the presence of both the amino and carboxyl groups, amino acids typically exist as zwitterions at physiological pH. A zwitterion is a molecule with both a positive and a negative charge, but with a net overall neutral charge. In amino acids, the amino group is protonated (+NH3), and the carboxyl group is deprotonated (-COO-). This zwitterionic form contributes significantly to the unique properties of amino acids, such as their high melting points and solubility in water.
Importance of Amino and Carboxyl Groups in Protein Structure and Function
The amino and carboxyl groups are not merely structural components; they are crucial for the diverse functions and structures exhibited by proteins. Their roles extend beyond peptide bond formation:
- Protein Folding: The interactions between amino and carboxyl groups of different amino acids, as well as their interactions with the surrounding environment (water molecules, ions, etc.), guide the folding process of proteins. Hydrogen bonding, electrostatic interactions, and hydrophobic interactions all contribute to the formation of the protein's unique three-dimensional structure.
- Protein-Protein Interactions: The charged nature of the amino and carboxyl groups allows for interactions with other molecules, including other proteins. These interactions are essential for signaling pathways, enzymatic catalysis, and the formation of protein complexes.
- Enzyme Activity: The amino and carboxyl groups often participate directly in catalytic reactions. They can act as acid-base catalysts, donating or accepting protons to facilitate chemical transformations.
- Binding Sites: The amino and carboxyl groups frequently form part of binding sites on proteins, enabling them to interact specifically with substrates, ligands, or other molecules.
Beyond the Two Core Groups: The Side Chain's Influence
While the amino and carboxyl groups are common to all amino acids, the side chain (R group) distinguishes one amino acid from another, granting them a wide range of properties. The side chain's chemical nature influences the amino acid’s polarity, charge, and interactions with other molecules. Some side chains are nonpolar and hydrophobic, while others are polar or even charged, affecting the protein's overall solubility and interactions.
The interplay between the core amino and carboxyl groups and the diverse side chains creates a vast array of protein structures and functions. The specific amino acid sequence, determined by genetic information, dictates the protein's three-dimensional structure, influencing its interactions and biological roles.
Conclusion
The presence of both the amino (-NH2) and carboxyl (-COOH) groups is the defining characteristic of amino acids. These two functional groups are indispensable for peptide bond formation, the basic process underlying protein synthesis. Beyond their roles in constructing the polypeptide chain, these groups are crucial for determining the properties of amino acids, influencing protein folding, stability, and interactions. Their inherent chemical properties – basicity and acidity, respectively – along with their capacity for hydrogen bonding and diverse chemical modifications, contribute substantially to the remarkable diversity and biological functions of proteins, the essential molecules for life. Understanding the profound implications of these two functional groups is crucial for comprehending the complexities of biochemistry and molecular biology.
Latest Posts
Latest Posts
-
Rounding Percentages To The Nearest Tenth
Mar 19, 2025
-
What Happens To Acceleration When Mass Is Doubled
Mar 19, 2025
-
The Hottest Layer Of The Earth Is The
Mar 19, 2025
-
Whats The Difference Between Rough Er And Smooth Er
Mar 19, 2025
-
How Much Atp Is Produced In The Electron Transport Chain
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Which Two Functional Groups Are Always Found In Amino Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
