Which Particles Account For The Mass Of The Atom
Juapaving
Mar 17, 2025 · 6 min read
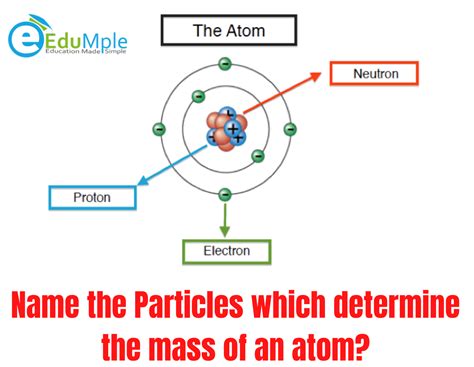
Table of Contents
Which Particles Account for the Mass of the Atom?
The seemingly simple question, "Which particles account for the mass of an atom?" delves into the fascinating world of quantum mechanics and reveals a surprising answer. While the intuitive response might point towards protons and neutrons, the reality is far more nuanced and involves a deeper understanding of mass-energy equivalence and the fundamental forces governing the subatomic realm. This article will explore the contributions of different particles to atomic mass, examining the roles of protons, neutrons, and electrons, and clarifying the misconceptions surrounding this topic.
The Dominant Players: Protons and Neutrons
The majority of an atom's mass resides within its nucleus, a tiny, dense core containing protons and neutrons. These particles are collectively known as nucleons. Let's delve into their individual contributions:
Protons: The Positively Charged Nucleus
Protons are positively charged particles with a mass approximately 1836 times greater than that of an electron. Their charge (+1) determines the atomic number of an element, defining its unique chemical properties. While their mass is significantly larger than that of electrons, it's relatively small compared to the overall mass of the atom, especially in heavier atoms. However, their contribution is undeniable and crucial to the atom's overall identity.
Neutrons: The Neutral Mass Contributors
Neutrons, as their name suggests, carry no electrical charge. Their mass is very similar to that of protons, slightly larger, and plays a crucial role in stabilizing the atom's nucleus. The number of neutrons in an atom can vary, even within the same element, leading to the existence of isotopes. Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. For instance, Carbon-12 and Carbon-14 are isotopes of carbon, differing only in their neutron count (6 and 8 respectively). The neutrons' significant mass contribution is directly proportional to the atom's overall mass. Their presence is key to nuclear stability, preventing the repulsive forces between protons from tearing the nucleus apart.
The Negligible Electron Mass
Electrons, orbiting the nucleus, possess a tiny mass, approximately 1/1836th the mass of a proton. Consequently, their contribution to the total mass of an atom is essentially negligible. While electrons play a pivotal role in chemical reactions and determine an atom's chemical behavior, they contribute almost insignificantly to its overall mass. For all practical purposes, when calculating atomic mass, the electron mass can be ignored.
Mass-Energy Equivalence: E=mc²
The famous equation by Einstein, E=mc², profoundly impacts our understanding of mass. It reveals that energy and mass are interchangeable. The mass of a particle isn't solely an intrinsic property but also encompasses the energy associated with its internal structure and the binding energies holding the nucleus together. This insight further clarifies the contributions of protons and neutrons to the atom's mass.
Binding Energy: The Nuclear Glue
The strong nuclear force binds protons and neutrons together within the nucleus. This force is incredibly strong at short distances but diminishes rapidly with increasing separation. The process of forming the nucleus from individual nucleons releases a significant amount of energy, known as binding energy. This energy, according to Einstein's equation, is equivalent to a small decrease in mass. Therefore, the actual mass of a nucleus is slightly less than the sum of the masses of its constituent protons and neutrons. This mass defect directly relates to the strong nuclear binding energy. The larger the binding energy, the greater the mass defect, and hence, a smaller overall mass than expected.
Rest Mass vs. Relativistic Mass
It is important to differentiate between rest mass and relativistic mass. Rest mass is the mass of a particle at rest, while relativistic mass increases with velocity. At everyday speeds, the relativistic mass increase is negligible. However, at speeds approaching the speed of light, this effect becomes significant. In the context of atomic mass, we typically consider rest mass, as the speeds of electrons and nucleons within the atom are far below the speed of light.
Beyond Protons and Neutrons: A Deeper Dive
While protons and neutrons account for the bulk of an atom's mass, a more precise understanding requires delving into the composition of these particles themselves. Protons and neutrons are not fundamental particles; they are composed of even smaller particles called quarks.
Quarks: The Fundamental Building Blocks
Quarks are fundamental particles that interact through the strong nuclear force, mediated by gluons. There are six types of quarks: up, down, charm, strange, top, and bottom. Protons consist of two up quarks and one down quark, while neutrons consist of one up quark and two down quarks. The masses of these quarks contribute to the overall mass of the proton and neutron, though it's important to note that the masses of the constituent quarks alone do not entirely account for the mass of the proton and neutron.
Gluons and the Strong Force: Energy Contributing to Mass
The strong force, holding quarks together within protons and neutrons, is mediated by gluons. Gluons themselves possess energy, and, as per E=mc², this energy contributes to the mass of the protons and neutrons. The energy of the strong force's interactions significantly contributes to the nucleon's mass – a key factor often overlooked in simplistic explanations. This is a crucial point that helps explain why the sum of the quark masses alone doesn't equal the proton or neutron mass.
Isotopes and Atomic Mass: Averages and Variations
The atomic mass of an element listed in the periodic table is typically a weighted average of the masses of its naturally occurring isotopes. Since isotopes of the same element have different numbers of neutrons, they possess slightly different masses. The weighted average considers the relative abundance of each isotope in nature. This average reflects the overall mass distribution found in a naturally occurring sample of that element.
Conclusion: A Holistic Perspective
The seemingly straightforward question regarding the particles responsible for an atom's mass reveals the intricate nature of matter at the subatomic level. While protons and neutrons, through their constituent quarks and the energy associated with the strong nuclear force, account for the vast majority of an atom's mass, the electron's contribution is negligible. Understanding mass-energy equivalence and the complexities of nuclear binding energy is crucial to grasping the holistic picture. This exploration highlights the rich interconnectedness of concepts in physics and the ever-evolving nature of our understanding of the universe's fundamental building blocks. From the macroscopic world to the subatomic realm, the principles of physics remain consistent, revealing an intricate dance of forces and particles that ultimately shape the universe as we know it. Further research continues to refine our knowledge of these fundamental interactions, promising even deeper insights into the composition of matter in the future.
Latest Posts
Latest Posts
-
What Is 25 In A Decimal
Mar 18, 2025
-
The Scattering Of Light By Colloids Is Called
Mar 18, 2025
-
Least Common Multiple Of 2 4 And 8
Mar 18, 2025
-
How Many Equal Sides Does An Isosceles Triangle Have
Mar 18, 2025
-
Full Form Of S I T
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Which Particles Account For The Mass Of The Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
