The Scattering Of Light By Colloids Is Called
Juapaving
Mar 18, 2025 · 6 min read
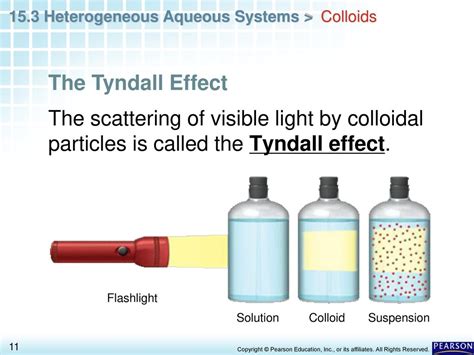
Table of Contents
The Scattering of Light by Colloids is Called Tyndall Effect: A Deep Dive
The scattering of light by colloids is a phenomenon known as the Tyndall effect. This effect, named after the 19th-century physicist John Tyndall, is a crucial aspect of understanding the optical properties of colloidal dispersions and has far-reaching applications in various scientific fields. This article will delve into the intricacies of the Tyndall effect, explaining its underlying principles, factors influencing its intensity, and its significance in different contexts.
Understanding Colloids and their Optical Behavior
Before diving into the specifics of the Tyndall effect, let's establish a firm understanding of colloids. Colloids are heterogeneous mixtures where one substance is dispersed evenly throughout another. The dispersed substance, known as the dispersed phase, consists of particles significantly larger than those found in true solutions (typically ranging from 1 to 1000 nanometers in diameter). These particles, however, are smaller than those in suspensions, which are large enough to settle out of the solution over time. Common examples of colloids include milk, fog, ink, and blood.
Unlike true solutions, where the solute particles are too small to scatter visible light, the larger particles in colloids interact differently with light. This interaction is primarily responsible for the characteristic appearance of colloidal dispersions. The key difference lies in the size of the particles relative to the wavelength of visible light. Particles smaller than the wavelength of light tend to undergo Rayleigh scattering, where the scattered light is predominantly blue. Larger particles, characteristic of colloids, exhibit a more complex scattering pattern, leading to the Tyndall effect.
The Tyndall Effect: A Detailed Explanation
The Tyndall effect is the scattering of light as a light beam passes through a colloid. This scattering results in the beam becoming visible. The scattered light’s intensity depends on several factors, including the wavelength of the light, the size and concentration of the colloidal particles, and the refractive index difference between the dispersed phase and the dispersion medium.
How it Works: When a beam of light encounters a colloidal particle, it interacts with the particle's electrons. This interaction causes the electrons to oscillate, re-radiating light in all directions. This re-radiated light constitutes the scattered light. The intensity of the scattering is inversely proportional to the fourth power of the wavelength (λ⁻⁴). This explains why shorter wavelengths (blue light) are scattered more strongly than longer wavelengths (red light), a phenomenon also observed in Rayleigh scattering, but the overall scattering effect is significantly more pronounced in the Tyndall effect.
Key Differences between Tyndall Effect and Rayleigh Scattering
While both Tyndall effect and Rayleigh scattering involve the scattering of light, there are important distinctions:
- Particle Size: Rayleigh scattering occurs with particles significantly smaller than the wavelength of light, whereas the Tyndall effect involves particles comparable to or larger than the wavelength of light.
- Wavelength Dependence: Both exhibit wavelength dependence, but the Tyndall effect shows a less pronounced dependence on wavelength compared to Rayleigh scattering. While blue light is still preferentially scattered, the effect is not as dramatically blue-shifted as in Rayleigh scattering.
- Scattering Intensity: The Tyndall effect generally results in a higher intensity of scattered light than Rayleigh scattering due to the larger size of the scattering particles.
- Polarization: The scattered light in the Tyndall effect is generally partially polarized, while Rayleigh scattering produces fully polarized light at right angles to the incident beam.
Factors Affecting the Tyndall Effect
Several factors influence the intensity and visibility of the Tyndall effect:
- Particle Size: Larger particles scatter more light. As particle size increases, so does the intensity of the scattered light. This is because larger particles interact more strongly with the incident light wave.
- Particle Concentration: A higher concentration of colloidal particles leads to a more intense Tyndall effect due to a larger number of scattering centers.
- Wavelength of Light: Shorter wavelengths (blue light) are scattered more effectively than longer wavelengths (red light), although the effect is less dramatic than in Rayleigh scattering.
- Refractive Index Difference: A larger difference in refractive index between the dispersed phase and the dispersion medium results in a more pronounced Tyndall effect. The greater the difference, the stronger the interaction between light and the particles.
Applications of the Tyndall Effect
The Tyndall effect has numerous applications across various fields:
1. Determining Colloidal Nature:
The Tyndall effect serves as a simple test to differentiate between true solutions and colloidal dispersions. If a light beam passes through a solution and remains invisible, it indicates a true solution. However, if the beam becomes visible due to scattering, it points to a colloidal dispersion.
2. Atmospheric Science:
The Tyndall effect plays a crucial role in determining the visibility of the atmosphere. Scattering of sunlight by atmospheric aerosols and dust particles causes the characteristic blue color of the sky during the day and the reddish hues of sunsets and sunrises. The presence of pollutants or other aerosols can significantly affect the Tyndall effect and thus reduce visibility.
3. Medical Diagnostics:
The Tyndall effect is used in some medical diagnostic techniques. For example, the Tyndall effect can help in identifying the presence of inflammation in the eye. The presence of colloids in bodily fluids can also be detected using this phenomenon.
4. Material Science:
The Tyndall effect finds applications in material science for the characterization of various materials, including nanoparticles and polymers. The intensity of scattered light can provide insights into particle size distribution and other material properties.
5. Photography and Lighting:
The Tyndall effect is utilized in photography and lighting to create specific visual effects. For instance, the scattering of light by small particles in the air can be used to create a hazy or dreamy atmosphere in photographs.
Beyond the Basics: Advanced Concepts
The Tyndall effect is a complex phenomenon that involves several advanced concepts in physics and chemistry. Here are a few areas that delve deeper into its intricate nature:
- Mie Scattering: For larger particles, the scattering behavior deviates significantly from Rayleigh scattering and becomes more accurately described by Mie scattering theory. This theory takes into account the particle size and refractive index more accurately.
- Dynamic Light Scattering (DLS): DLS is a technique that utilizes the fluctuation in the intensity of scattered light to determine the size and size distribution of colloidal particles.
- Light Scattering Spectroscopy: This technique uses the wavelength dependence of scattered light to obtain detailed information about the properties of the scattering particles.
Conclusion: The Significance of Tyndall Effect
The Tyndall effect is a fundamental phenomenon in colloid science with far-reaching implications. Its understanding is crucial in various fields, from atmospheric science and medical diagnostics to material science and photography. The ability to observe and quantify the Tyndall effect provides invaluable insights into the properties of colloidal dispersions and the interactions between light and matter at the nanoscale. Further research into the intricacies of the Tyndall effect continues to unlock new applications and enhance our understanding of this fascinating optical phenomenon. The continued investigation of the Tyndall effect, particularly using advanced techniques like DLS and light scattering spectroscopy, promises to reveal even more about the behavior of colloids and the properties of light and matter at the nanoscale. This deeper understanding has the potential to further advance fields ranging from materials science and medical diagnostics to environmental monitoring and climate modeling. Thus, the study of the Tyndall effect is not merely an academic pursuit; it holds considerable practical significance and continues to be a vibrant area of ongoing research.
Latest Posts
Latest Posts
-
How Many Protons Neutrons And Electrons In Sodium
Mar 18, 2025
-
What Are Factor Pairs Of 30
Mar 18, 2025
-
Which Chamber Of The Heart Has The Thickest Muscular Wall
Mar 18, 2025
-
What Is The Largest Cell On Earth
Mar 18, 2025
-
What Is 2 5 As A Percentage
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about The Scattering Of Light By Colloids Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
