Which Of The Following Is An Element
Juapaving
Mar 30, 2025 · 7 min read
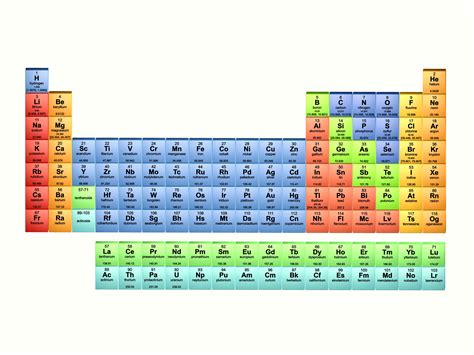
Table of Contents
Which of the Following is an Element? Understanding the Building Blocks of Matter
Understanding the fundamental building blocks of matter is crucial in various fields, from chemistry and physics to materials science and engineering. At the heart of this understanding lies the concept of an element – a pure substance that cannot be broken down into simpler substances by chemical means. This article delves deep into the definition of an element, explores how it differs from compounds and mixtures, and provides numerous examples to solidify your understanding. We will also touch upon the periodic table, the ultimate organizational tool for all known elements.
Defining an Element: The Purest Form of Matter
An element is defined as a substance consisting of only one type of atom. An atom, in turn, is the smallest unit of an element that retains the chemical properties of that element. It's important to emphasize the word "chemical" here. While atoms can be further broken down into subatomic particles (protons, neutrons, and electrons), this process involves nuclear reactions, not chemical reactions. Chemical reactions involve the rearrangement of atoms, not their fundamental alteration.
Think of elements as the ultimate Lego bricks of the universe. You can combine these bricks in various ways to build complex structures (compounds and mixtures), but each brick itself remains fundamentally unchanged.
Key Characteristics of Elements:
- Pure Substance: Elements are pure substances, meaning they are composed of only one type of atom. This homogeneity is a defining characteristic.
- Indivisible by Chemical Means: Elements cannot be broken down into simpler substances through chemical reactions such as burning, dissolving, or reacting with acids or bases. Only nuclear reactions can alter the composition of an element.
- Unique Properties: Each element possesses a unique set of chemical and physical properties, such as melting point, boiling point, density, reactivity, and conductivity. These properties allow us to distinguish one element from another.
- Represented by Symbols: Elements are represented by chemical symbols, usually one or two letters derived from their name (e.g., H for hydrogen, O for oxygen, Fe for iron).
Distinguishing Elements from Compounds and Mixtures
Understanding elements requires differentiating them from compounds and mixtures. These are all forms of matter, but their compositions differ significantly:
Compounds: A Chemical Combination
A compound is a substance formed when two or more different elements are chemically bonded together in a fixed ratio. This bonding creates a new substance with properties distinctly different from its constituent elements. For example, water (H₂O) is a compound formed from the elements hydrogen and oxygen. The properties of water are vastly different from those of hydrogen gas and oxygen gas. The ratio of hydrogen to oxygen in water is always 2:1. This fixed ratio is a critical characteristic of compounds. Compounds can be broken down into their constituent elements through chemical means.
Mixtures: A Physical Combination
A mixture is a combination of two or more substances that are not chemically bonded. Mixtures can be homogeneous (uniform composition throughout, like saltwater) or heterogeneous (non-uniform composition, like sand and water). The components of a mixture retain their individual properties. Unlike compounds, mixtures can be separated into their components through physical methods such as filtration, distillation, or evaporation. There's no fixed ratio of components in a mixture.
The Periodic Table: Organizing the Elements
The periodic table is a tabular arrangement of all known chemical elements, organized by their atomic number, electron configuration, and recurring chemical properties. Elements are arranged in rows (periods) and columns (groups or families) based on their electron configurations. Elements within the same group exhibit similar chemical properties. The periodic table is an indispensable tool for chemists, physicists, and material scientists. It allows us to predict the properties of elements based on their position in the table and to understand the relationships between elements.
Key features of the Periodic Table:
- Atomic Number: The atomic number represents the number of protons in an atom's nucleus. This number uniquely identifies each element.
- Periods: Horizontal rows represent periods, with elements in the same period having the same number of electron shells.
- Groups/Families: Vertical columns represent groups or families, with elements in the same group sharing similar chemical properties due to similar outer electron configurations.
- Metals, Nonmetals, and Metalloids: The periodic table categorizes elements into metals (typically shiny, conductive, malleable), nonmetals (generally brittle, poor conductors), and metalloids (elements with properties intermediate between metals and nonmetals).
Examples of Elements and Their Applications
Let's explore several common elements and their widespread applications:
1. Oxygen (O): The Breath of Life
Oxygen is a crucial element for respiration in most living organisms. It's also used in various industrial processes, including welding, metal fabrication, and water treatment. Oxygen is a highly reactive element, readily forming compounds with other elements.
2. Hydrogen (H): The Lightest Element
Hydrogen is the lightest and most abundant element in the universe. It's used as a fuel in fuel cells and rockets, and it's a crucial component in the production of ammonia (used in fertilizers) and methanol (used as a solvent and fuel additive).
3. Carbon (C): The Basis of Life
Carbon is the backbone of organic chemistry, forming the basis of all living organisms. It's also essential in various materials, including diamonds, graphite, and fullerenes. The unique bonding capabilities of carbon allow for the formation of a vast number of organic compounds.
4. Iron (Fe): A Metal of Strength
Iron is a strong, relatively inexpensive metal used extensively in construction, manufacturing, and transportation. It's also a vital component of hemoglobin, the protein that carries oxygen in our blood.
5. Gold (Au): A Precious Metal
Gold is a precious metal valued for its beauty, resistance to corrosion, and excellent conductivity. It's used in jewelry, electronics, and investments.
6. Silicon (Si): The Heart of Electronics
Silicon is a crucial element in the electronics industry, forming the basis of semiconductors used in computer chips, solar cells, and other electronic devices.
7. Nitrogen (N): Essential for Life
Nitrogen is a major component of the atmosphere and is essential for plant growth. It's also used in the production of fertilizers and explosives.
8. Chlorine (Cl): Disinfectant and More
Chlorine is a highly reactive nonmetal used as a disinfectant in water treatment and swimming pools. It's also used in the production of various chemicals.
Identifying Elements: Techniques and Methods
Identifying an element involves determining its unique atomic number and, consequently, its chemical identity. Several techniques are employed for this purpose:
1. Spectroscopy: Analyzing Light
Spectroscopy involves analyzing the light emitted or absorbed by an element. Each element possesses a unique spectral fingerprint, allowing for its identification. Atomic absorption spectroscopy (AAS) and atomic emission spectroscopy (AES) are commonly used techniques.
2. Chromatography: Separating Mixtures
Chromatography is a technique used to separate mixtures into their individual components. This separation allows for the identification of elements present in a sample. Gas chromatography (GC) and high-performance liquid chromatography (HPLC) are widely used methods.
3. Mass Spectrometry: Determining Isotopic Ratios
Mass spectrometry measures the mass-to-charge ratio of ions. This technique is used to determine the isotopic ratios of an element, further aiding in its identification. Different isotopes of an element have the same number of protons but different numbers of neutrons.
4. X-ray Diffraction: Analyzing Crystal Structures
X-ray diffraction is used to analyze the crystal structure of a material. The diffraction pattern is unique to each element, allowing for its identification. This technique is particularly useful for solid materials.
Conclusion: The Importance of Understanding Elements
Understanding elements, their properties, and their interactions is fundamental to numerous scientific disciplines. From the development of new materials and technologies to comprehending the intricacies of life itself, the knowledge of elements provides a crucial foundation. The periodic table serves as a powerful tool for organizing and understanding these fundamental building blocks of matter, while various analytical techniques allow us to identify and characterize elements in diverse samples. The journey into the world of elements is a journey into the very fabric of our universe.
Latest Posts
Latest Posts
-
Common Denominator For 3 And 4
Apr 01, 2025
-
How To Spell 20 In Word Form
Apr 01, 2025
-
Anything That Has Mass And Occupies Space Is Defined As
Apr 01, 2025
-
Is Soda A Mixture Or Compound
Apr 01, 2025
-
How Much Sides Does A Octagon Have
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is An Element . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
