Anything That Has Mass And Occupies Space Is Defined As
Juapaving
Apr 01, 2025 · 7 min read
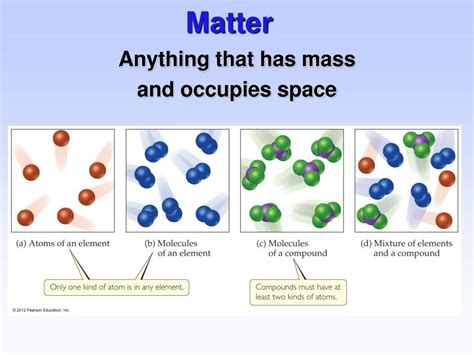
Table of Contents
- Anything That Has Mass And Occupies Space Is Defined As
- Table of Contents
- Anything That Has Mass and Occupies Space is Defined as Matter: A Deep Dive into the Building Blocks of the Universe
- What is Matter? A Closer Examination
- The Three States of Matter: Solid, Liquid, and Gas
- Beyond the Three States: Plasma and Bose-Einstein Condensates
- The Building Blocks of Matter: Atoms and Molecules
- Properties of Matter: Physical and Chemical Properties
- The Fundamental Forces Governing Matter
- Changes in Matter: Physical and Chemical Changes
- The Conservation of Mass and Energy
- Conclusion: An Ongoing Exploration
- Latest Posts
- Latest Posts
- Related Post
Anything That Has Mass and Occupies Space is Defined as Matter: A Deep Dive into the Building Blocks of the Universe
The simple statement, "anything that has mass and occupies space is defined as matter," forms the bedrock of our understanding of the physical world. This seemingly straightforward definition opens a vast and fascinating landscape of scientific exploration, encompassing everything from the smallest subatomic particles to the largest celestial bodies. This article will delve into the intricacies of matter, exploring its various forms, properties, and the fundamental forces that govern its behavior.
What is Matter? A Closer Examination
At its core, matter is anything that possesses both mass and volume. Mass refers to the amount of matter an object contains, often measured in kilograms or grams. Volume, on the other hand, represents the amount of three-dimensional space an object occupies, typically measured in cubic meters or liters. This definition effectively distinguishes matter from energy, which, while possessing energy, doesn't occupy space in the same way.
While seemingly simple, this definition encompasses a stunning diversity of substances. From the air we breathe to the ground we walk on, from the water we drink to the stars we gaze upon, everything observable in the universe is composed of matter in some form. Understanding the properties and behaviors of matter is fundamental to comprehending the workings of the universe.
The Three States of Matter: Solid, Liquid, and Gas
Matter exists primarily in three fundamental states: solid, liquid, and gas. These states are defined by the arrangement and interaction of the particles (atoms and molecules) that make up the matter.
-
Solids: In solids, particles are tightly packed together in a fixed, ordered arrangement. This gives solids their characteristic rigidity and definite shape and volume. The particles vibrate in place but do not move freely. Examples include rocks, ice, and wood. The strong intermolecular forces hold the particles firmly in place.
-
Liquids: Liquids possess a definite volume but no fixed shape. Their particles are closely packed but are not held in a rigid arrangement. This allows liquids to flow and take the shape of their container. Particles in liquids move more freely than in solids but are still relatively close together. Examples include water, oil, and mercury.
-
Gases: Gases have neither a definite shape nor a definite volume. Their particles are widely dispersed and move freely and rapidly in all directions. This allows gases to expand to fill any container they occupy. The weak intermolecular forces allow for extensive movement and separation of the particles. Examples include air, oxygen, and helium.
Beyond the Three States: Plasma and Bose-Einstein Condensates
While solids, liquids, and gases are the most commonly observed states of matter, two additional states deserve mention:
-
Plasma: Plasma is often described as an ionized gas, meaning that its atoms have lost or gained electrons, resulting in a mixture of ions and free electrons. This state is characterized by high temperatures and electrical conductivity. Plasma is the most common state of matter in the universe, making up stars and much of interstellar space. Examples include lightning, neon signs, and the sun.
-
Bose-Einstein Condensates (BECs): At extremely low temperatures, close to absolute zero, certain types of atoms can form a BEC. In this state, a large fraction of the atoms occupy the lowest quantum state, behaving as a single, macroscopic quantum entity. BECs exhibit remarkable properties, including superfluidity and macroscopic quantum phenomena.
The Building Blocks of Matter: Atoms and Molecules
Matter is composed of fundamental units called atoms. Atoms are incredibly tiny, consisting of a central nucleus containing protons and neutrons, surrounded by a cloud of orbiting electrons. The number of protons in an atom's nucleus determines its atomic number and defines the element it represents. Elements are the basic building blocks of all matter. For example, hydrogen (H), oxygen (O), and carbon (C) are elements.
Atoms can combine chemically to form molecules. Molecules are formed when atoms share or transfer electrons to achieve a more stable electronic configuration. The properties of a molecule are often significantly different from the properties of the constituent atoms. For instance, two hydrogen atoms combine to form a molecule of hydrogen gas (H₂), a gas, while oxygen atoms combine to form oxygen gas (O₂), also a gas. Water (H₂O) is another example of a molecule formed by the combination of hydrogen and oxygen atoms.
Properties of Matter: Physical and Chemical Properties
Matter possesses a range of properties, which can be broadly classified as physical and chemical.
Physical Properties: These properties can be observed or measured without changing the chemical composition of the matter. Examples include:
- Color: The visual appearance of the substance.
- Density: The mass per unit volume.
- Melting point: The temperature at which a solid transforms into a liquid.
- Boiling point: The temperature at which a liquid transforms into a gas.
- Solubility: The ability of a substance to dissolve in another.
- Conductivity: The ability to conduct electricity or heat.
Chemical Properties: These properties describe how a substance reacts with other substances, involving a change in its chemical composition. Examples include:
- Flammability: The ability to burn in the presence of oxygen.
- Reactivity: How readily a substance reacts with other substances.
- Toxicity: The degree to which a substance is poisonous.
- Acidity/Basicity (pH): A measure of the concentration of hydrogen ions in a solution.
The Fundamental Forces Governing Matter
The behavior of matter is governed by four fundamental forces:
-
Strong Nuclear Force: This force holds the protons and neutrons together within the atomic nucleus, overcoming the electrostatic repulsion between the positively charged protons.
-
Electromagnetic Force: This force governs the interactions between electrically charged particles. It is responsible for the attraction between electrons and the nucleus, as well as interactions between molecules.
-
Weak Nuclear Force: This force is responsible for certain types of radioactive decay, involving the transformation of one type of subatomic particle into another.
-
Gravitational Force: This force is the weakest of the four fundamental forces, but it acts over vast distances, attracting all matter towards each other. It is responsible for the formation of planets, stars, and galaxies.
Changes in Matter: Physical and Chemical Changes
Matter can undergo two main types of changes: physical and chemical.
Physical Changes: These changes alter the form or appearance of matter but do not change its chemical composition. Examples include:
- Changes in state (melting, freezing, boiling, condensation): These changes involve a transition between the different states of matter without altering the chemical makeup.
- Dissolving: A substance dissolving in a solvent does not change its chemical composition; it simply changes its physical state.
- Crushing or breaking: These actions alter the physical form but not the chemical makeup.
Chemical Changes (Chemical Reactions): These changes involve a rearrangement of atoms and molecules, resulting in the formation of new substances with different properties. Examples include:
- Burning (combustion): This reaction involves the rapid combination of a substance with oxygen, producing heat and light.
- Rusting (oxidation): This reaction involves the slow combination of a metal with oxygen, forming a metal oxide.
- Digestion: The breaking down of food molecules into smaller molecules.
The Conservation of Mass and Energy
A cornerstone of physics is the principle of the conservation of mass and energy. This principle states that mass and energy cannot be created or destroyed, only transformed from one form to another. Einstein's famous equation, E=mc², illustrates the equivalence of mass and energy, where energy (E) is equal to mass (m) multiplied by the speed of light (c) squared. This equation shows that a small amount of mass can be converted into a tremendous amount of energy, as seen in nuclear reactions.
Conclusion: An Ongoing Exploration
The definition of matter as anything possessing mass and occupying space provides a foundational understanding of the physical world. However, the study of matter is a dynamic and evolving field. Ongoing research continues to refine our understanding of the fundamental building blocks of matter, the forces that govern their behavior, and the diverse forms that matter can take. From the complexities of quantum mechanics to the vastness of astrophysics, the study of matter remains a central theme in scientific inquiry, revealing ever more about the universe we inhabit. Further exploration into fields like nanotechnology, material science, and cosmology promises to reveal even deeper insights into the nature of matter and its profound impact on the world around us.
Latest Posts
Latest Posts
-
Electron Configuration For A Neutral Atom Of Oxygen
Apr 05, 2025
-
What Is The Lcm Of 16 And 12
Apr 05, 2025
-
Which Of The Following Statements Are False
Apr 05, 2025
-
What Type Of Medium Travels The Slowest
Apr 05, 2025
-
Find The Least Common Multiple Of 5 And 3
Apr 05, 2025
Related Post
Thank you for visiting our website which covers about Anything That Has Mass And Occupies Space Is Defined As . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
