Which Of The Following Is An Amino Group
Juapaving
Mar 21, 2025 · 6 min read
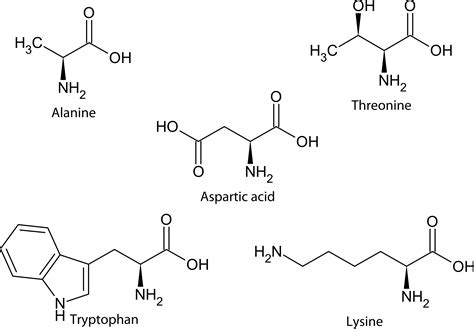
Table of Contents
Which of the Following is an Amino Group? A Deep Dive into Amino Acids and their Functional Groups
Understanding amino acids and their constituent parts is fundamental to comprehending the building blocks of life. Proteins, the workhorses of biological systems, are constructed from chains of amino acids, each uniquely defined by its side chain and, crucially, its characteristic amino group. This article delves into the intricacies of amino groups, clarifying their structure, function, and importance in the context of amino acids and broader biochemistry.
What is an Amino Group?
An amino group is a functional group characterized by a nitrogen atom bonded to two hydrogen atoms and a carbon atom (–NH₂). Its chemical formula is –NH₂. This seemingly simple structure is responsible for a plethora of crucial chemical reactions and properties within biological molecules. The nitrogen atom possesses a lone pair of electrons, making it a strong nucleophile – it readily donates electrons to other atoms or molecules. This nucleophilic nature is pivotal to the reactivity of amino acids and peptides.
The amino group is basic, meaning it can readily accept a proton (H⁺) to become positively charged (–NH₃⁺). This property dictates the behavior of amino acids at different pH levels, impacting their solubility and their ability to participate in various biochemical processes. This protonation/deprotonation equilibrium is fundamental to understanding protein structure and function.
Amino Acids: The Building Blocks of Life
Amino acids are organic molecules that serve as the fundamental units of proteins. Each amino acid comprises three key components:
- A central carbon atom (α-carbon): This carbon atom acts as the backbone, connecting the other three components.
- An amino group (–NH₂): As discussed above, this is a basic functional group.
- A carboxyl group (–COOH): This is an acidic functional group, capable of donating a proton (H⁺).
- A side chain (R group): This is the variable part of the amino acid, giving each amino acid its unique properties. The R group can be aliphatic, aromatic, polar, or non-polar.
The diversity of R groups accounts for the twenty standard amino acids that make up the majority of proteins. These amino acids can be broadly categorized based on the properties of their R groups, further influencing the overall protein structure and function.
The Importance of the Amino Group in Amino Acid Chemistry
The amino group plays a critical role in several key aspects of amino acid chemistry:
-
Peptide Bond Formation: The amino group of one amino acid reacts with the carboxyl group of another amino acid to form a peptide bond. This is a crucial condensation reaction that links amino acids together to create polypeptide chains, which fold and assemble into functional proteins. The peptide bond formation involves the elimination of a water molecule and the formation of a C-N bond.
-
Acid-Base Properties: The amino group’s ability to accept a proton determines the amino acid's overall charge and its behavior in solutions of different pH. At physiological pH (around 7.4), the amino group is generally protonated (–NH₃⁺), contributing to the overall positive charge of the amino acid. This property significantly affects the solubility and interaction of amino acids within the cellular environment.
-
Enzyme Catalysis: The amino group can participate directly in enzymatic reactions as a nucleophile or a general base. This involves its lone pair of electrons participating in the catalytic mechanism. Many enzymes utilize amino acid side chains, including the amino group, to facilitate chemical reactions.
-
Protein Folding and Structure: The charge and hydrogen bonding capabilities of the amino group greatly influence the three-dimensional structure of proteins. Hydrogen bonds involving the amino group contribute to the secondary structure elements such as alpha-helices and beta-sheets.
-
Post-Translational Modifications: Amino groups can undergo post-translational modifications, which alter the properties of the protein. For instance, acetylation, a common modification, involves the addition of an acetyl group to the amino group, often affecting the protein's stability and function.
Distinguishing the Amino Group from Other Functional Groups
It’s important to be able to distinguish the amino group from other functional groups with similar appearances, such as:
-
Amide Group (–CONH₂): While structurally similar, the amide group contains a carbonyl group (C=O) bonded to the nitrogen atom. This carbonyl group significantly alters its reactivity and properties compared to the amino group. Amide groups are found in peptide bonds.
-
Imine Group (–C=NH): This group contains a carbon-nitrogen double bond, rather than the single bond found in the amino group. Imines exhibit different chemical properties due to the double bond's influence.
-
Nitro Group (–NO₂): The nitro group contains a nitrogen atom bonded to two oxygen atoms. Its presence significantly alters the chemical properties of the molecule, unlike the amino group.
The subtle differences in structure between these functional groups have profound consequences for their reactivity and the properties of the molecules they constitute. Understanding these differences is crucial for accurately identifying and characterizing biomolecules.
Amino Groups in Non-Protein Molecules
Amino groups aren't limited to amino acids and proteins. They also appear in other biologically important molecules:
-
Nucleobases: Certain nucleobases, like adenine and guanine, which are essential components of DNA and RNA, contain amino groups. These amino groups contribute to the hydrogen bonding interactions crucial for the base-pairing of DNA and RNA.
-
Neurotransmitters: Some neurotransmitters, chemical messengers in the nervous system, contain amino groups. For example, dopamine and serotonin possess amino groups that are crucial for their interaction with receptors and their function in the brain.
-
Many other metabolites: countless metabolic intermediates and products of various metabolic pathways contain amino groups playing critical roles in their function within the organism.
Identifying an Amino Group in a Chemical Structure
Identifying an amino group within a larger chemical structure requires careful observation:
- Look for a nitrogen atom: The amino group always contains a nitrogen atom.
- Check for two hydrogen atoms bonded to the nitrogen: These hydrogen atoms are a key characteristic of the amino group.
- Consider the bonding environment: The nitrogen atom in an amino group is typically bonded to a carbon atom (–NH₂-C), forming a part of a larger molecule.
Practical Applications and Future Directions
The understanding of amino groups and their functions has led to various practical applications in diverse fields. The development of novel drugs, materials science, and synthetic biology hinges on a thorough understanding of the chemistry of amino groups. Future research focuses on exploring the diversity of amino group modification and its potential for engineering proteins with tailored functionalities. This includes the development of novel biosensors and therapeutic agents based on amino group-specific reactions.
In summary, the amino group is a fundamental functional group with far-reaching implications in biochemistry and beyond. Its role in amino acid chemistry, protein structure, and various other biological processes underlines its importance in the maintenance of life itself. A thorough understanding of its structure and properties is crucial for advancements in various scientific disciplines.
Latest Posts
Latest Posts
-
What Quality Is Notable About The Stratum Corneum
Mar 28, 2025
-
What Is The Role Of Toothpaste In Preventing Cavities
Mar 28, 2025
-
The Reaction Between An Organic Acid And An Alcohol Produces
Mar 28, 2025
-
Tap Water Mixture Or Pure Substance
Mar 28, 2025
-
The Role Of The Light Harvesting Complex Is To
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is An Amino Group . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
