Tap Water Mixture Or Pure Substance
Juapaving
Mar 28, 2025 · 6 min read
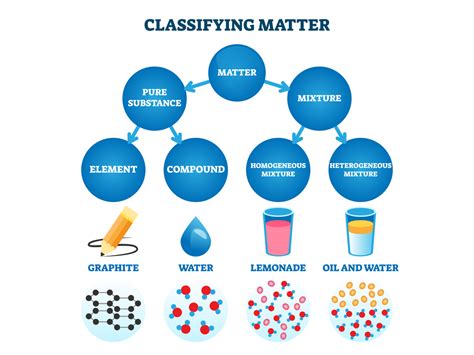
Table of Contents
Tap Water: Mixture or Pure Substance? Delving into the Composition of Everyday Water
Water. We drink it, bathe in it, use it to cook – it's fundamental to life. But have you ever stopped to consider what's actually in your tap water? Is it a pure substance, or a complex mixture? The answer, as with many scientific questions, is nuanced. This comprehensive guide will explore the fascinating composition of tap water, clarifying its status as a mixture and examining the various substances it contains. We'll also explore the implications of this composition on human health and the environment.
Understanding Pure Substances and Mixtures
Before we delve into the complexities of tap water, let's establish a clear understanding of the fundamental difference between pure substances and mixtures.
Pure substance: A pure substance is a form of matter that has a constant chemical composition and properties throughout a given sample. It cannot be separated into components by physical means. Examples include elements (like oxygen or gold) and compounds (like water, H₂O, in its purest form).
Mixture: A mixture is a combination of two or more substances that are not chemically bonded. The components of a mixture retain their individual properties, and they can be separated by physical means like filtration, distillation, or evaporation. Tap water is a prime example of a mixture.
The Composition of Tap Water: A Complex Mixture
Tap water is far from pure H₂O. Its composition varies considerably depending on its source (river, lake, aquifer), the treatment processes employed, and the location. However, we can generally categorize the components of tap water into several main groups:
1. Water (H₂O): The Primary Component
While not a pure substance in tap water, H₂O still makes up the vast majority of its volume, typically exceeding 99%. This water, however, has already undergone several processes and is far from the pristine H₂O molecule found in a chemistry lab.
2. Dissolved Minerals: Essential and Trace Elements
Tap water naturally dissolves minerals from the earth as it travels through underground aquifers or surface water sources. These minerals can include:
- Calcium (Ca²⁺): Essential for bone health and other bodily functions. High levels can contribute to scale buildup in pipes and appliances.
- Magnesium (Mg²⁺): Another essential mineral vital for various metabolic processes.
- Sodium (Na⁺): While necessary in small amounts, high sodium levels can be a concern for individuals with hypertension.
- Potassium (K⁺): An important electrolyte for proper nerve and muscle function.
- Bicarbonates (HCO₃⁻): Contribute to the water's alkalinity and buffering capacity.
- Sulfates (SO₄²⁻): Can impart a slightly bitter taste at high concentrations.
- Chlorides (Cl⁻): Contribute to the water's salinity.
- Iron (Fe²⁺, Fe³⁺): Can cause discoloration and staining if present in high levels.
- Manganese (Mn²⁺): Similar to iron, excess manganese can lead to discoloration.
- Fluoride (F⁻): Often added to tap water to prevent tooth decay. The concentration is carefully regulated to balance benefits and potential risks.
The Importance of Mineral Content: While some minerals are beneficial, others can impart undesirable taste or even have adverse health effects at high concentrations. The mineral content of tap water contributes to its "hardness," influencing its suitability for certain applications like washing clothes or brewing coffee. Water that is very high in minerals can cause scale buildup in appliances, whereas soft water can lead to increased corrosion.
3. Dissolved Gases: Oxygen, Carbon Dioxide, and Others
Gases from the atmosphere, like oxygen and carbon dioxide, can dissolve into tap water. The concentration of these gases can vary depending on the water's exposure to the atmosphere and its temperature. These gases often play a role in the water's taste and its ability to support aquatic life.
4. Disinfectants: Ensuring Safe Drinking Water
To prevent the spread of waterborne diseases, tap water is usually disinfected. Common disinfectants include:
- Chlorine (Cl₂): A widely used and effective disinfectant that kills bacteria and viruses. While effective, it can impart a noticeable chlorine taste and odor to the water. Some people find this unpleasant, while others may experience allergic reactions.
- Chloramines (NH₂Cl): A combination of chlorine and ammonia, often used as a secondary disinfectant. They provide longer-lasting disinfection but can also produce undesirable tastes and odors.
- Ozone (O₃): A powerful disinfectant that doesn't leave a lingering taste or odor. However, it's more expensive and less stable than chlorine.
- Ultraviolet (UV) light: UV light is used to kill microorganisms without adding chemicals to the water.
5. Byproducts of Disinfection: Unintended Consequences
The disinfection process itself can create byproducts, some of which are potentially harmful. These byproducts are called disinfection byproducts (DBPs). Examples include:
- Trihalomethanes (THMs): These are formed when chlorine reacts with organic matter in the water. Some THMs are suspected carcinogens.
- Haloacetic acids (HAAs): Similar to THMs, these are also formed during chlorination and are potential health concerns.
Regulations are in place to limit the concentration of these DBPs in tap water to minimize potential risks.
6. Other Potential Contaminants: A Wide Range of Substances
Depending on the source and treatment processes, tap water may contain other substances, including:
- Pesticides: Runoff from agricultural fields can contaminate water sources.
- Pharmaceuticals and Personal Care Products (PPCPs): These are increasingly detected in tap water, raising concerns about their long-term effects.
- Industrial pollutants: Depending on the location, industrial discharge can contaminate water sources with various pollutants.
- Heavy metals: Lead, mercury, and other heavy metals can leach into water from pipes or industrial sources. Lead contamination is a particular concern, especially in older homes with lead pipes.
The Impact of Tap Water Composition on Human Health and the Environment
The composition of tap water has significant implications for both human health and the environment. The presence of beneficial minerals can support our bodily functions, but excessive concentrations of certain minerals or contaminants can have adverse effects.
Human Health: Consuming water with high levels of sodium, heavy metals, or DBPs can pose significant health risks. Long-term exposure can increase the risk of various health problems, including cardiovascular disease, cancer, and neurological disorders. Regular monitoring and stringent regulations are crucial to ensure safe drinking water.
Environment: The discharge of treated wastewater can have environmental consequences. Disinfectants and DBPs can impact aquatic ecosystems, potentially harming fish and other organisms. Excessive nutrients, such as nitrates from fertilizers, can contribute to eutrophication (algal blooms) in water bodies, leading to oxygen depletion and harming aquatic life.
Conclusion: Tap Water – A Complex Mixture Requiring Careful Management
Tap water is unequivocally a mixture, not a pure substance. Its composition is complex and variable, influenced by numerous factors. While it's a vital resource providing essential minerals and supporting human life, its potential to contain undesirable substances necessitates continuous monitoring, stringent regulations, and advanced water treatment technologies to protect public health and the environment. Understanding the composition of our tap water allows us to appreciate the sophisticated systems in place to provide safe drinking water, while also highlighting the need for continued research and responsible stewardship of this precious resource. The ongoing challenge is to balance the benefits of tap water with the potential risks posed by contaminants and byproducts of treatment. Understanding this complexity empowers informed decision-making regarding water consumption and environmental protection.
Latest Posts
Latest Posts
-
Differences Between Renewable And Nonrenewable Resources
Mar 31, 2025
-
Is 16 A Prime Or Composite
Mar 31, 2025
-
Whats The Square Root Of 8
Mar 31, 2025
-
Which Element Is The Most Reactive Metal
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Tap Water Mixture Or Pure Substance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
