The Reaction Between An Organic Acid And An Alcohol Produces
Juapaving
Mar 28, 2025 · 6 min read
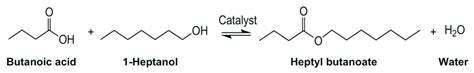
Table of Contents
The Reaction Between an Organic Acid and an Alcohol: Esterification Unveiled
The reaction between an organic acid and an alcohol is a cornerstone of organic chemistry, resulting in the formation of an ester and water. This process, known as esterification, is a crucial reaction with widespread applications in various industries, from the production of fragrances and flavors to the synthesis of pharmaceuticals and polymers. Understanding the mechanism, influencing factors, and applications of this reaction is key to appreciating its significance in chemistry and beyond.
Understanding Esterification: A Detailed Look
Esterification is a reversible reaction, meaning it can proceed in both the forward (ester formation) and reverse (ester hydrolysis) directions. The forward reaction involves the nucleophilic attack of the alcohol's oxygen atom on the carbonyl carbon of the carboxylic acid. This process, often catalyzed by an acid, leads to the formation of an ester and a water molecule.
The Mechanism: A Step-by-Step Approach
The mechanism of acid-catalyzed esterification can be broken down into several key steps:
-
Protonation of the carbonyl oxygen: The acid catalyst, typically a strong acid like sulfuric acid or hydrochloric acid, protonates the carbonyl oxygen of the carboxylic acid. This increases the electrophilicity of the carbonyl carbon, making it more susceptible to nucleophilic attack.
-
Nucleophilic attack by the alcohol: The oxygen atom of the alcohol, acting as a nucleophile, attacks the electrophilic carbonyl carbon. This forms a tetrahedral intermediate.
-
Proton transfer: A proton is transferred from the hydroxyl group of the tetrahedral intermediate to one of the oxygen atoms, leading to the formation of a neutral molecule with a good leaving group.
-
Elimination of water: The protonated hydroxyl group leaves as a water molecule, regenerating the carbonyl group. This step forms the ester.
-
Deprotonation: The protonated ester is deprotonated by a base (often the conjugate base of the acid catalyst), yielding the final ester product.
Key Players: Carboxylic Acids and Alcohols
The success of esterification hinges on the properties of the reacting carboxylic acid and alcohol. Carboxylic acids, characterized by the -COOH functional group, provide the carbonyl carbon for nucleophilic attack. Their reactivity can be influenced by factors like steric hindrance (bulky groups near the carboxyl group) and electronic effects (electron-donating or withdrawing groups).
Alcohols, featuring the -OH functional group, act as nucleophiles, donating their oxygen's lone pair of electrons to initiate the reaction. Similar to carboxylic acids, their reactivity is affected by steric hindrance and electronic effects. Primary alcohols generally react faster than secondary alcohols, while tertiary alcohols often show poor reactivity in esterification.
Factors Affecting Esterification: Optimization for Success
Several factors significantly influence the yield and rate of esterification. Understanding and controlling these factors is crucial for optimizing the reaction.
Acid Catalyst: The Driving Force
The presence of an acid catalyst is essential for efficient esterification. The catalyst protonates the carbonyl oxygen, enhancing its electrophilicity and accelerating the reaction. The choice of catalyst depends on the specific reactants and desired reaction conditions. Stronger acids generally lead to faster reaction rates.
Temperature: Balancing Speed and Equilibrium
Temperature plays a crucial role in the equilibrium position and reaction rate. Higher temperatures generally increase the reaction rate, but they also shift the equilibrium towards the reactants (Le Chatelier's principle). Finding the optimal temperature involves balancing speed and yield.
Reaction Time: Patience Yields Results
Sufficient reaction time is necessary for the reaction to reach equilibrium or a desired conversion. Monitoring the reaction's progress using techniques like titration or chromatography helps determine the optimal reaction time.
Water Removal: Shifting Equilibrium
Since esterification is a reversible reaction, removing the water formed during the process drives the equilibrium towards ester formation. This can be achieved through techniques like azeotropic distillation (removing a water-organic solvent azeotrope), using a desiccant, or employing techniques like Dean-Stark apparatus.
Steric Hindrance: Bulky Groups and Reactivity
Steric hindrance, caused by bulky substituents on either the carboxylic acid or alcohol, can significantly reduce the reaction rate. Large groups around the reaction centers hinder the approach of the nucleophile and the subsequent steps of the mechanism.
Applications of Esterification: A Vast and Varied Landscape
The versatility of esterification makes it a widely utilized reaction in various fields.
Flavor and Fragrance Industry: The Allure of Esters
Esters are responsible for the characteristic aromas and flavors of many fruits, flowers, and other natural products. Esterification is used extensively in the flavor and fragrance industry to synthesize esters with specific desirable odors and tastes, used in perfumes, cosmetics, and food products. For example, ethyl acetate contributes to the aroma of many fruits, while methyl salicylate is the primary component of wintergreen oil.
Polymer Chemistry: Building Blocks of Macromolecules
Esterification plays a vital role in polymer chemistry. Polyesters, a large class of polymers, are synthesized via the polymerization of dicarboxylic acids and dialcohols. These polymers find wide application in various materials like clothing fibers (polyester), packaging films, and medical implants.
Pharmaceutical Industry: Tailoring Medicinal Compounds
Esterification is employed extensively in the pharmaceutical industry to modify the properties of drug molecules. Esterification can improve the drug's solubility, absorption, and bioavailability, leading to more effective medications. For example, many aspirin formulations utilize acetylsalicylic acid, an ester derivative of salicylic acid.
Other Applications: Beyond the Main Sectors
Beyond the major applications, esterification has found uses in diverse areas like:
- Biodiesel production: Transesterification, a type of esterification, converts vegetable oils and animal fats into biodiesel, a renewable fuel.
- Coatings and paints: Esterification is used to synthesize various resins and coatings, providing durability and protection to surfaces.
- Lubricant synthesis: Ester-based lubricants are used in various applications due to their superior properties compared to petroleum-based lubricants.
Variations and Related Reactions: Expanding the Scope
Several variations and related reactions expand upon the core principles of esterification:
Transesterification: An Exchange of Esters
Transesterification involves the exchange of one alcohol for another in an ester molecule. This reaction is widely used in the production of biodiesel, where vegetable oils or animal fats (containing triglycerides) react with methanol to yield methyl esters (biodiesel) and glycerol.
Fischer Esterification: The Classic Approach
Fischer esterification is a classic method for synthesizing esters using a carboxylic acid and an alcohol in the presence of an acid catalyst. It is a widely used and versatile method for ester synthesis.
Enzymatic Esterification: Biocatalysis in Action
Enzymatic esterification uses enzymes, typically lipases, to catalyze the reaction. This approach offers several advantages, including milder reaction conditions, higher selectivity, and reduced waste generation. It finds increasing use in producing specific esters with high purity.
Conclusion: A Reaction of Profound Impact
The reaction between an organic acid and an alcohol, leading to the formation of an ester, is a fundamental and widely applied reaction in organic chemistry. Understanding the mechanism, factors influencing the reaction, and its diverse applications is crucial for chemists and researchers across various fields. From the production of fragrances to the synthesis of pharmaceuticals and polymers, esterification continues to play a vital role in shaping our world, showcasing the power of fundamental chemical reactions. Continued research and development in this area promise even further advancements and applications in the future. The ever-expanding landscape of esterification underscores its enduring significance in chemical synthesis and beyond.
Latest Posts
Latest Posts
-
Least Common Multiple Of 20 And 15
Mar 31, 2025
-
The Horizontal Rows In The Periodic Table Are Called
Mar 31, 2025
-
How Many Meters In 7 Feet
Mar 31, 2025
-
How Is A Square Similar To A Rhombus
Mar 31, 2025
-
Work Done For The Process Shown In The Figure Is
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about The Reaction Between An Organic Acid And An Alcohol Produces . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
