Which Of The Following Is A True Solution
Juapaving
Mar 15, 2025 · 5 min read
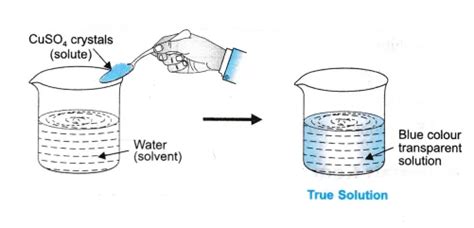
Table of Contents
Which of the Following is a True Solution? Understanding Solution Types in Chemistry
The question, "Which of the following is a true solution?" often appears in chemistry exams and requires a deep understanding of solution chemistry. It's not just about memorizing definitions; it's about grasping the underlying principles that differentiate true solutions from other mixtures. This comprehensive guide will delve into the various types of mixtures, focusing on the characteristics that define a true solution and how to distinguish it from colloids and suspensions. We'll also explore the factors influencing solubility and the applications of true solutions in various fields.
Defining a True Solution
A true solution is a homogeneous mixture composed of two or more substances. Crucially, the solute (the substance being dissolved) is completely dissolved in the solvent (the substance doing the dissolving) at the molecular level. This means the particles of the solute are individually dispersed throughout the solvent, resulting in a uniform composition throughout the mixture. This uniformity is a key distinguishing feature. You cannot see the individual components of a true solution with the naked eye; they're uniformly distributed.
Key Characteristics of a True Solution:
- Homogeneous: The composition is uniform throughout. No matter where you take a sample from the solution, it will have the same concentration of solute.
- Particle Size: The solute particles are extremely small, typically less than 1 nanometer (nm) in diameter. They are at the atomic or molecular level, invisible even under a powerful microscope.
- Filtration: A true solution cannot be separated by simple filtration. The solute particles are too small to be trapped by filter paper.
- Sedimentation: The solute will not settle out of the solution over time due to gravity. The particles are too small to be affected significantly by gravity.
- Transparency: True solutions are usually transparent. Light passes through them without significant scattering. This is because the solute particles are too small to scatter light effectively.
Distinguishing True Solutions from Colloids and Suspensions
Understanding the differences between true solutions, colloids, and suspensions is crucial for answering the question accurately. These are all mixtures, but they differ significantly in the size of their dispersed particles.
Colloids
Colloids, also known as colloidal dispersions, are mixtures where the dispersed particles are larger than those in a true solution but smaller than those in a suspension. These particles typically range from 1 nm to 1000 nm in diameter. They are too small to be seen with the naked eye, but they scatter light, a phenomenon known as the Tyndall effect.
Examples of Colloids:
- Milk
- Fog
- Blood
- Ink
- Gelatin
Key Characteristics of Colloids:
- Heterogeneous (at the microscopic level): While appearing homogeneous to the naked eye, colloids are heterogeneous at the microscopic level. The dispersed particles are larger than in a true solution.
- Particle Size: 1 nm to 1000 nm
- Tyndall Effect: Scatter light, resulting in a cloudy or hazy appearance.
- Filtration: Cannot be separated by simple filtration.
- Sedimentation: Particles do not settle out over time.
Suspensions
Suspensions are heterogeneous mixtures containing relatively large particles that are visible to the naked eye. These particles are typically larger than 1000 nm in diameter. They settle out of the mixture over time due to gravity.
Examples of Suspensions:
- Muddy water
- Sand in water
- Flour in water
Key Characteristics of Suspensions:
- Heterogeneous: The composition is not uniform throughout.
- Particle Size: Larger than 1000 nm
- Sedimentation: Particles settle out over time.
- Filtration: Can be separated by simple filtration.
- Appearance: Usually opaque or cloudy.
Factors Affecting Solubility and True Solution Formation
The ability of a solute to dissolve in a solvent to form a true solution depends on several factors:
- Nature of the Solute and Solvent: "Like dissolves like" is a crucial principle. Polar solvents (e.g., water) tend to dissolve polar solutes (e.g., sugar, salt), while nonpolar solvents (e.g., oil) dissolve nonpolar solutes (e.g., fats, oils).
- Temperature: Increasing the temperature usually increases the solubility of solids in liquids. However, the effect of temperature on gas solubility is often the opposite; increasing temperature decreases the solubility of gases in liquids.
- Pressure: Pressure has a significant effect on the solubility of gases in liquids. Increasing pressure increases the solubility of gases. This is why carbonated beverages are bottled under pressure.
- Concentration: The maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature is called its solubility. A solution that contains the maximum amount of solute is called a saturated solution. A solution that contains less than the maximum amount of solute is called an unsaturated solution. A supersaturated solution contains more solute than it can normally hold at that temperature.
Applications of True Solutions
True solutions are ubiquitous in various fields, including:
- Medicine: Many medications are administered as true solutions for efficient absorption by the body.
- Industry: Chemical processes often involve true solutions for precise control of reactant concentrations.
- Agriculture: Fertilizers are often applied as true solutions for optimal nutrient uptake by plants.
- Food and Beverages: Many food and beverages are true solutions, providing a homogeneous distribution of flavors and nutrients.
- Environmental Science: Understanding the solubility of pollutants in water is crucial for assessing environmental risks.
Identifying a True Solution: A Practical Approach
When faced with the question, "Which of the following is a true solution?", systematically consider the characteristics discussed above:
- Examine the Appearance: Is the mixture homogeneous and transparent, or is it cloudy or opaque? A cloudy or opaque mixture is unlikely to be a true solution.
- Consider the Particle Size: Can you see individual particles with the naked eye or a microscope? If so, it's probably not a true solution.
- Test for Filtration: Can you separate the components by simple filtration? If yes, it's not a true solution.
- Observe Sedimentation: Does the mixture settle over time? If so, it's definitely not a true solution.
- Check for the Tyndall Effect: Does the mixture scatter light? The Tyndall effect indicates a colloid, not a true solution.
By carefully analyzing these aspects, you can accurately identify which of the given options represents a true solution. Remember that understanding the underlying principles of solution chemistry is paramount to mastering this concept and answering such questions confidently. The ability to distinguish true solutions from other types of mixtures is a fundamental skill in chemistry with wide-ranging applications in various fields. Therefore, mastering this knowledge is not merely beneficial for academic pursuits but also crucial for real-world applications across numerous scientific and technological disciplines.
Latest Posts
Latest Posts
-
How Many Symmetry Lines Does A Square Have
Mar 17, 2025
-
Do Viruses Belong To One Of The Domains Of Life
Mar 17, 2025
-
The C Shape Of The Tracheal Cartilages Is Important Because
Mar 17, 2025
-
Least Common Multiple Of 5 6 7
Mar 17, 2025
-
How Do You Find The Inverse Of A Relation
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is A True Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
