Which Of The Following Is A Property Of An Acid
Juapaving
Mar 19, 2025 · 7 min read
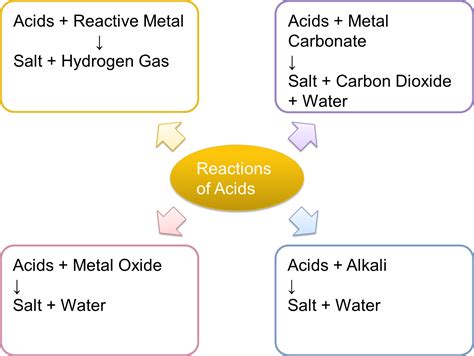
Table of Contents
Which of the Following is a Property of an Acid? A Comprehensive Guide
Acids are ubiquitous in our lives, from the citric acid in oranges to the sulfuric acid used in car batteries. Understanding their properties is crucial in various fields, from chemistry and biology to environmental science and even cooking. This comprehensive guide delves into the defining characteristics of acids, exploring their properties in detail and clarifying common misconceptions. We'll examine several key properties and discuss how they are measured and applied.
Defining Acids: More Than Just a Sour Taste
While the sour taste of many acids is a familiar characteristic, it's far from the only defining property. The most widely accepted definition of an acid relies on its behavior in chemical reactions. According to the Brønsted-Lowry theory, an acid is a proton (H⁺) donor. This means it readily donates a hydrogen ion (a proton) to another substance, called a base. The Brønsted-Lowry theory provides a broader understanding of acidity than older definitions, as it encompasses a wider range of substances.
Another important definition is the Lewis theory of acids and bases. A Lewis acid is defined as an electron pair acceptor. This definition expands the concept of acidity to include substances that don't necessarily contain hydrogen but can still accept electron pairs, forming coordinate covalent bonds. While both theories are valuable, the Brønsted-Lowry definition is more commonly used when discussing the properties we’ll examine here.
Key Properties of Acids
Let's delve into the specific properties that characterize acids:
1. Sour Taste: A Sensory Indicator (But Use Caution!)
While not a scientific definition, the sour taste is a characteristic many people associate with acids. Citric acid in citrus fruits, acetic acid in vinegar, and lactic acid in sour milk all exemplify this property. However, it's crucial to emphasize that tasting chemicals to identify them is extremely dangerous and should never be attempted. Many acids are corrosive and can cause severe damage to the mouth, throat, and esophagus.
2. pH Less Than 7: The Quantitative Measure of Acidity
The pH scale provides a quantitative measurement of acidity and alkalinity. The scale ranges from 0 to 14, with 7 representing neutrality. Acids have a pH value less than 7. The lower the pH value, the stronger the acid. For example, stomach acid has a pH around 1.5, while vinegar has a pH around 3. The pH scale is logarithmic, meaning each whole number change represents a tenfold change in hydrogen ion concentration. Therefore, a solution with a pH of 2 is ten times more acidic than a solution with a pH of 3.
3. Reaction with Metals: Producing Hydrogen Gas
Acids react with many metals, producing hydrogen gas (H₂) and a salt. This is a classic chemical reaction used to identify acids. For example, the reaction between hydrochloric acid (HCl) and zinc (Zn) produces zinc chloride (ZnCl₂) and hydrogen gas:
2HCl(aq) + Zn(s) → ZnCl₂(aq) + H₂(g)
This reaction is often observed as bubbling or fizzing when an acid is added to a reactive metal. The hydrogen gas produced can be tested using a burning splint, which will ignite with a squeaky pop sound. This reaction is not applicable to all metals; some metals, like gold and platinum, are unreactive with most acids.
4. Reaction with Bases: Neutralization Reactions
Acids react with bases in a process called neutralization. This reaction produces water and a salt. For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) produces sodium chloride (NaCl) and water (H₂O):
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
Neutralization reactions are frequently used in titrations to determine the concentration of an acid or base. The endpoint of the titration, where the acid and base have completely reacted, is often indicated by a change in color using an indicator.
5. Reaction with Carbonates and Bicarbonates: Producing Carbon Dioxide
Acids react with carbonates (CO₃²⁻) and bicarbonates (HCO₃⁻) to produce carbon dioxide (CO₂), water, and a salt. This reaction is often observed as bubbling or fizzing. For example, the reaction between hydrochloric acid (HCl) and sodium carbonate (Na₂CO₃) produces sodium chloride (NaCl), carbon dioxide (CO₂), and water (H₂O):
2HCl(aq) + Na₂CO₃(s) → 2NaCl(aq) + CO₂(g) + H₂O(l)
This reaction is commonly used in the production of carbonated beverages and as a test for the presence of carbonates. The carbon dioxide gas can be collected and tested.
6. Electrical Conductivity: Strong vs. Weak Acids
Acids can conduct electricity, but the extent of conductivity varies depending on their strength. Strong acids, such as hydrochloric acid (HCl) and sulfuric acid (H₂SO₄), completely dissociate into ions in aqueous solutions, resulting in high electrical conductivity. Weak acids, such as acetic acid (CH₃COOH) and carbonic acid (H₂CO₃), only partially dissociate, resulting in lower electrical conductivity. The degree of dissociation determines the concentration of ions in solution and, consequently, the conductivity.
7. Indicators: Visual Confirmation of Acidity
Acid-base indicators are substances that change color depending on the pH of the solution. These indicators are often used in titrations to determine the endpoint of the neutralization reaction. Common indicators include litmus paper, which turns red in acidic solutions and blue in alkaline solutions, and phenolphthalein, which is colorless in acidic solutions and pink in alkaline solutions. Different indicators change color at different pH ranges, allowing for precise determination of the pH.
8. Corrosiveness: A Dangerous Property
Many acids are corrosive, meaning they can damage or destroy other materials through chemical reactions. Strong acids are particularly corrosive and can cause severe burns to skin and eyes. The corrosive nature of acids is due to their ability to react with many substances, including organic tissues. Always handle acids with appropriate safety precautions, including wearing gloves, eye protection, and working in a well-ventilated area.
Strong vs. Weak Acids: A Key Distinction
It's essential to differentiate between strong acids and weak acids. Strong acids completely ionize in water, meaning all their molecules dissociate into ions. Examples include hydrochloric acid (HCl), sulfuric acid (H₂SO₄), and nitric acid (HNO₃). Weak acids only partially ionize, meaning only a small fraction of their molecules dissociate into ions. Examples include acetic acid (CH₃COOH), carbonic acid (H₂CO₃), and citric acid (C₆H₈O₇). This difference in ionization affects their pH, reactivity, and other properties.
Applications of Acids: A Wide Range of Uses
Acids play vital roles in numerous applications across various industries:
- Food and Beverage Industry: Citric acid is used as a flavoring agent and preservative. Acetic acid is the main component of vinegar. Lactic acid contributes to the sour taste of yogurt and other fermented foods.
- Pharmaceutical Industry: Acids are used in the synthesis of many drugs and medications. They also act as catalysts in various chemical reactions used in drug manufacturing.
- Industrial Processes: Sulfuric acid is a key industrial chemical used in the production of fertilizers, detergents, and other products. Hydrochloric acid is used in metal cleaning and pickling.
- Environmental Applications: Acids are used in water treatment to adjust pH and remove impurities. They also play a role in soil acidification, affecting plant growth and nutrient availability.
Conclusion: Understanding the Properties of Acids
Understanding the properties of acids is fundamental in various fields. From their sour taste and pH values to their reactions with metals, bases, and carbonates, each property provides insight into their chemical behavior. Remembering the safety precautions associated with handling acids is paramount. The distinction between strong and weak acids further adds to the complexity and importance of this fundamental chemical concept. By understanding these properties, we can appreciate the significant role acids play in our daily lives and various industries. This knowledge is crucial for safe handling, effective application, and a deeper understanding of the chemical world around us.
Latest Posts
Latest Posts
-
Which Is Greater 1 3 Or 2 5
Mar 19, 2025
-
Gay Lussacs Law Real Life Example
Mar 19, 2025
-
What Are 2 Kingdoms Of Bacteria
Mar 19, 2025
-
What Is The Most Reactive Group Of Nonmetals
Mar 19, 2025
-
How Does Nadp Turn Into Nadph
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is A Property Of An Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
