Gay- Lussac's Law Real Life Example
Juapaving
Mar 19, 2025 · 5 min read
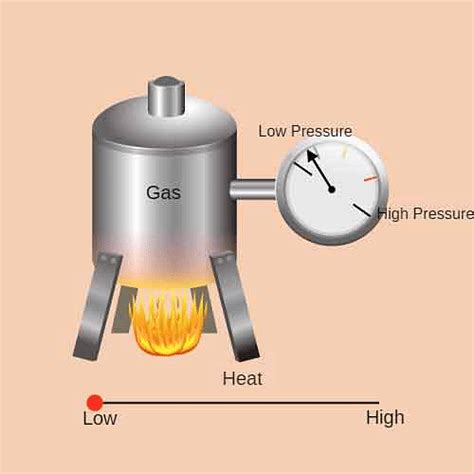
Table of Contents
Gay-Lussac's Law: Real-Life Examples and Applications
Gay-Lussac's Law, also known as Amontons's Law, is a fundamental gas law that describes the relationship between the pressure and temperature of a gas when the volume is held constant. Understanding this law is crucial in various fields, from everyday life to complex industrial processes. This article delves deep into Gay-Lussac's Law, exploring its principle, mathematical representation, and a wide range of compelling real-life examples.
Understanding Gay-Lussac's Law: The Basics
Gay-Lussac's Law states that the pressure of a given amount of gas held at constant volume is directly proportional to the absolute temperature. In simpler terms, as the temperature of a gas increases, its pressure also increases proportionally, provided the volume remains unchanged. Conversely, if the temperature decreases, the pressure will decrease proportionally.
This relationship can be mathematically expressed as:
P₁/T₁ = P₂/T₂
Where:
- P₁ represents the initial pressure of the gas
- T₁ represents the initial absolute temperature of the gas (in Kelvin)
- P₂ represents the final pressure of the gas
- T₂ represents the final absolute temperature of the gas (in Kelvin)
It's crucial to remember that temperature must always be expressed in Kelvin (K) for Gay-Lussac's Law calculations. Kelvin is an absolute temperature scale where 0 K represents absolute zero, the theoretical point where all molecular motion ceases. To convert Celsius (°C) to Kelvin (K), simply add 273.15: K = °C + 273.15.
Real-Life Examples of Gay-Lussac's Law in Action
Gay-Lussac's Law isn't just a theoretical concept; it's a powerful principle with significant implications in the real world. Let's explore several compelling examples:
1. Pressure Cookers
Pressure cookers are a prime example of Gay-Lussac's Law in action. These kitchen appliances work by trapping steam inside a sealed container. As the water inside boils, the temperature increases, and consequently, the pressure inside the cooker rises significantly. This increased pressure leads to a higher boiling point for the water, allowing food to cook faster and more efficiently. The safety valve on a pressure cooker is a crucial safety feature that releases excess pressure if the temperature (and therefore pressure) gets too high, preventing potential explosions.
2. Aerosol Cans
Aerosol cans utilize the principle of Gay-Lussac's Law. These cans contain a propellant gas under high pressure. When the valve is pressed, the gas expands rapidly, propelling the contents outwards. The pressure inside the can is directly related to its temperature. On hot days, the temperature inside the can increases, leading to increased pressure and a higher risk of explosion. This is why storing aerosol cans in cool, shaded areas is crucial for safety.
3. Hot Air Balloons
Hot air balloons are a spectacular demonstration of Gay-Lussac's Law. Heating the air inside the balloon causes the air to expand and become less dense than the surrounding cooler air. This difference in density creates buoyancy, allowing the balloon to rise. As the pilot cools the air within the balloon, the pressure decreases, causing the balloon to descend. The intricate balance of temperature and pressure directly dictates the balloon's altitude.
4. Tire Pressure and Temperature
The pressure inside car or bicycle tires increases as the temperature rises. On a hot summer day, the air inside the tires expands, leading to increased pressure. This can be dangerous, as excessive pressure can cause tire blowouts. Conversely, in extremely cold weather, tire pressure decreases. Regularly checking tire pressure is essential to ensure safe driving and cycling conditions, particularly when there are significant temperature fluctuations.
5. Weather Balloons
Weather balloons are another excellent illustration of this principle. These balloons are filled with a gas like helium or hydrogen. As the balloon ascends, the atmospheric pressure decreases. If the gas inside the balloon were at constant volume, the balloon would still obey Gay-Lussac's Law – the temperature would need to drop to maintain a pressure equilibrium with its surrounding environment. However, since the balloons are typically designed to expand as they ascend, changes in volume also play a factor. The combination of pressure, temperature and volume changes allows meteorologists to gather crucial atmospheric data.
6. Internal Combustion Engines
Internal combustion engines in vehicles rely heavily on the principles of Gay-Lussac's Law. The combustion of fuel within the engine cylinders produces extremely high temperatures and pressures, pushing the pistons and ultimately driving the vehicle. The pressure generated is directly proportional to the temperature resulting from the combustion process. Understanding this relationship is vital in engine design and performance optimization.
7. Industrial Processes
Many industrial processes utilize Gay-Lussac's Law. For instance, in chemical plants and refineries, controlling the pressure and temperature of gases is vital for safe and efficient operation. Monitoring temperature fluctuations and adjusting pressure accordingly prevents hazardous situations and ensures process optimization. High-pressure gas storage tanks often have safety mechanisms designed to account for the increase in pressure due to temperature variations.
Limitations of Gay-Lussac's Law
While Gay-Lussac's Law provides a valuable framework for understanding the relationship between pressure and temperature in gases, it's crucial to acknowledge its limitations:
-
Ideal Gas Assumption: Gay-Lussac's Law assumes the gas behaves ideally. Real gases, particularly at high pressures and low temperatures, deviate from ideal behavior due to intermolecular forces and molecular volume. At such conditions, more sophisticated equations of state, like the van der Waals equation, are necessary for accurate predictions.
-
Constant Volume: The law strictly applies only when the volume of the gas remains constant. Any changes in volume will affect the pressure-temperature relationship and invalidate the simple mathematical formula.
-
Fixed Amount of Gas: The law applies to a fixed amount of gas. Adding or removing gas from the system will disrupt the relationship and require recalculation.
Conclusion
Gay-Lussac's Law is a fundamental principle in physics and chemistry with far-reaching applications in various fields. From everyday household appliances like pressure cookers to sophisticated industrial processes, understanding this law is essential for safe and efficient operation. While the law relies on certain assumptions and has limitations, its practical relevance remains undeniable. Knowing its principle and limitations allows for a better understanding of how gases behave under varying conditions and facilitates safer and more efficient technological applications. Further research and exploration into more complex equations of state are needed for handling situations where ideal gas assumptions break down. But the foundation laid by Gay-Lussac's Law remains a cornerstone in the study of gases and their behavior.
Latest Posts
Latest Posts
-
Difference Between Amorphous Solid And Crystalline Solid
May 09, 2025
-
How Big Is 54 Inches In Feet
May 09, 2025
-
Does Maternal And Fetal Blood Mix
May 09, 2025
-
5 Letter Words Ending In T Containing L
May 09, 2025
-
Is Methyl The Most Stable Radical
May 09, 2025
Related Post
Thank you for visiting our website which covers about Gay- Lussac's Law Real Life Example . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.