How Does Nadp+ Turn Into Nadph
Juapaving
Mar 19, 2025 · 6 min read
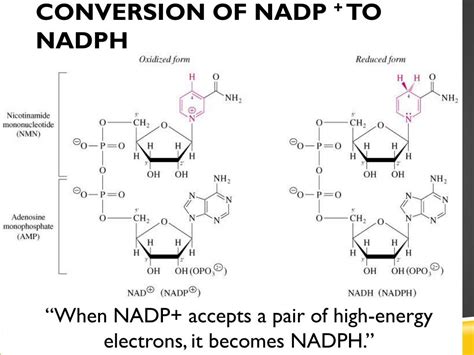
Table of Contents
How Does NADP+ Turn into NADPH? A Deep Dive into the Redox Reaction
The conversion of NADP+ to NADPH is a fundamental redox reaction crucial for countless metabolic processes within living organisms. Understanding this transformation is key to comprehending cellular energy production, biosynthesis, and the overall maintenance of cellular homeostasis. This article will delve into the intricate mechanisms involved, exploring the roles of enzymes, cofactors, and the broader context within metabolic pathways.
The Fundamentals: NADP+ and NADPH – A Redox Power Couple
Before we explore the conversion process, let's establish a foundational understanding of NADP+ and NADPH. Both are nucleotide coenzymes belonging to the vitamin B3 (niacin) family. They are essential electron carriers, participating in redox reactions by accepting or donating electrons.
-
NADP+ (Nicotinamide adenine dinucleotide phosphate): This is the oxidized form. It's ready to accept two electrons and one proton (H+), becoming reduced. Think of it as an electron sponge, eagerly waiting to soak up electrons.
-
NADPH (Nicotinamide adenine dinucleotide phosphate, reduced): This is the reduced form. It carries those two electrons and one proton, making it a potent electron donor. It readily releases these electrons to power various anabolic (biosynthetic) reactions.
The key difference lies in their reduction state: NADP+ is oxidized (lacking electrons), while NADPH is reduced (carrying electrons). This difference is crucial for their distinct roles in metabolism.
The Conversion: From NADP+ to NADPH – A Reductive Process
The transformation of NADP+ to NADPH is a reduction reaction. This means NADP+ gains electrons, becoming NADPH. This process doesn't happen spontaneously; it requires specific enzymes and often occurs within the context of larger metabolic pathways.
The core of the reaction involves the transfer of electrons and a proton to the nicotinamide ring of NADP+. This transfer alters the chemical structure, changing it from the positively charged NADP+ to the neutrally charged NADPH.
Key Players: Enzymes and their Mechanisms
Several enzymes catalyze the reduction of NADP+ to NADPH, depending on the specific metabolic context. These enzymes are generally dehydrogenases or reductases, indicating their role in the transfer of hydride ions (H⁻, which is a proton and two electrons).
Examples of enzymes involved in NADP+ reduction:
-
Glucose-6-phosphate dehydrogenase (G6PDH): This is a crucial enzyme in the pentose phosphate pathway (PPP). It catalyzes the first committed step in the PPP, oxidizing glucose-6-phosphate while simultaneously reducing NADP+ to NADPH. This pathway is primarily dedicated to producing NADPH and pentose sugars.
-
Malate dehydrogenase (NADP+-dependent): Found in various metabolic pathways, including the malate-aspartate shuttle, this enzyme catalyzes the oxidation of malate to oxaloacetate, concurrently reducing NADP+ to NADPH. This shuttle transports reducing equivalents (electrons) across the mitochondrial membrane.
-
Isocitrate dehydrogenase (NADP+-dependent): A key enzyme in the citric acid cycle (Krebs cycle), this isoform utilizes NADP+ as a cofactor, reducing it to NADPH during the oxidation of isocitrate to α-ketoglutarate.
These enzymes employ various mechanisms to facilitate the electron transfer. The detailed catalytic mechanisms vary, but the core principle remains the same: a substrate donates electrons to NADP+, leading to its reduction to NADPH.
Cofactors and Co-substrates: Essential Helpers
In many cases, the reduction of NADP+ isn't a direct transfer from a substrate molecule. Instead, it often involves intermediate electron carriers or co-substrates. These molecules act as bridges, facilitating the transfer of electrons from the initial substrate to NADP+.
For example, in the PPP, G6PDH uses the electrons from glucose-6-phosphate indirectly. The actual electron donor to NADP+ is often a tightly bound flavin adenine dinucleotide (FAD) or flavin mononucleotide (FMN) molecule within the enzyme's active site. These flavins are reduced by the substrate and then transfer electrons to NADP+.
The Role of Redox Potential
The conversion of NADP+ to NADPH is governed by the redox potential of the participating molecules. The redox potential measures a molecule's tendency to gain or lose electrons. NADP+ has a higher redox potential than NADPH, meaning NADP+ is more likely to accept electrons. The difference in redox potential provides the thermodynamic driving force for the reaction.
Metabolic Significance: Why is NADPH Crucial?
NADPH plays a vital role in a multitude of cellular processes, highlighting its importance as a key reducing agent:
1. Reductive Biosynthesis: NADPH is the primary electron donor in many anabolic pathways, fueling the synthesis of essential biomolecules such as:
- Fatty acids: The synthesis of fatty acids requires a significant amount of NADPH to reduce acetyl-CoA molecules, creating the hydrocarbon chains of fatty acids.
- Steroids: Steroid biosynthesis relies heavily on NADPH for reducing various intermediates in the complex pathways leading to cholesterol and other steroid hormones.
- Nucleotides: The building blocks of DNA and RNA, nucleotides, require NADPH for their synthesis.
- Amino acids: Some amino acid biosynthetic pathways utilize NADPH as a reducing agent in specific steps.
2. Antioxidant Defense: NADPH is crucial for the function of glutathione reductase, an enzyme that maintains the reduced form of glutathione (GSH). GSH is a powerful antioxidant, neutralizing reactive oxygen species (ROS) that can damage cellular components. NADPH replenishes the reduced form of glutathione, ensuring the cell's defense against oxidative stress.
3. Detoxification Reactions: Many detoxification pathways in the liver and other organs rely on NADPH to reduce harmful compounds, making them more water-soluble and easier to excrete. This is particularly important in removing xenobiotics (foreign substances) and toxic metabolites.
4. Pentose Phosphate Pathway (PPP): As mentioned earlier, the PPP primarily serves to generate NADPH and pentose sugars (five-carbon sugars) essential for nucleotide biosynthesis. The balanced production of both NADPH and pentose sugars highlights the integrated role of this pathway in anabolism.
Understanding NADP+/NADPH Ratio: A Cellular Balancing Act
The ratio of NADP+/NADPH within a cell is carefully regulated and reflects the cell's metabolic state. A high NADP+/NADPH ratio indicates a need for NADPH production, signaling a greater demand for reductive biosynthesis or antioxidant defense. Conversely, a low ratio suggests sufficient NADPH levels. Maintaining this balance is essential for cellular health and function. Disruptions in the NADP+/NADPH ratio can contribute to various pathological conditions.
Clinical Significance and Related Diseases
The importance of NADP+/NADPH balance extends to human health. Deficiencies in enzymes involved in NADPH production, such as G6PDH deficiency, can lead to hemolytic anemia due to impaired antioxidant defense. The resulting oxidative stress damages red blood cells, leading to their premature destruction.
Other conditions linked to dysregulation of NADPH metabolism include:
- Cancer: Altered NADPH metabolism is often observed in cancer cells, providing them with a proliferative advantage. Increased NADPH production fuels rapid cell growth and protects against oxidative stress-induced cell death.
- Inflammatory diseases: Imbalanced NADPH levels can contribute to inflammatory responses by affecting redox signaling and the production of ROS.
- Neurodegenerative diseases: Oxidative stress plays a significant role in neurodegenerative disorders, and impaired NADPH production could exacerbate neuronal damage.
Conclusion: A Cornerstone of Cellular Metabolism
The conversion of NADP+ to NADPH is not just a simple redox reaction; it's a critical process at the heart of cellular metabolism. This conversion provides the essential reducing power driving anabolic reactions, protecting cells from oxidative stress, and enabling various detoxification processes. The intricacy of the enzymes, co-factors, and regulatory mechanisms involved highlight the sophistication and importance of NADPH in maintaining cellular homeostasis and overall health. Further research continues to illuminate the profound implications of NADPH metabolism in health and disease, revealing new therapeutic targets and a deeper understanding of cellular function.
Latest Posts
Latest Posts
-
What Is The Prime Factorization Of 81
Mar 19, 2025
-
What Is The Least Common Multiple Of 2 And 3
Mar 19, 2025
-
Is 13 A Composite Or Prime Number
Mar 19, 2025
-
Compare And Contrast Active Transport And Facilitated Diffusion
Mar 19, 2025
-
How Many Cms Is 5 Feet
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Does Nadp+ Turn Into Nadph . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
