Which Of The Following Is A Characteristic Of Nonmetals
Juapaving
Mar 15, 2025 · 6 min read
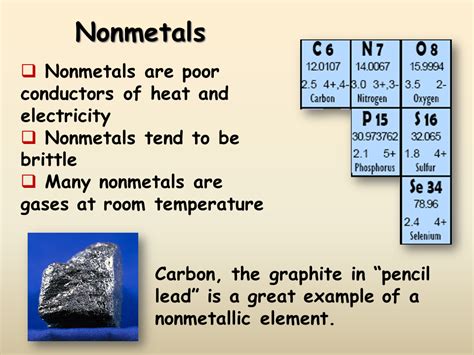
Table of Contents
Which of the Following is a Characteristic of Nonmetals? Exploring the Properties of Non-Metallic Elements
Understanding the properties of nonmetals is crucial for grasping fundamental concepts in chemistry. Unlike metals, which are known for their conductivity and malleability, nonmetals exhibit a distinct set of characteristics. This comprehensive guide delves into the key properties of nonmetals, providing a detailed analysis to help you confidently identify them. We will explore various physical and chemical properties, using examples to illustrate each characteristic.
Key Characteristics of Nonmetals
Nonmetals are a diverse group of elements found on the right-hand side of the periodic table. Their properties contrast sharply with those of metals, making identification relatively straightforward. Here are the defining characteristics:
1. Poor Conductors of Heat and Electricity
This is perhaps the most significant distinction between metals and nonmetals. Nonmetals are generally poor conductors of both heat and electricity. Electrons in nonmetals are tightly bound to their atoms, limiting their ability to move freely and carry charge. This contrasts sharply with metals, where electrons are delocalized and can flow easily. Think of the difference between a copper wire (metal, excellent conductor) and a rubber insulator (nonmetal, poor conductor).
Examples: Rubber, sulfur, oxygen, and chlorine are excellent examples of poor electrical and thermal conductors.
2. Brittle and Non-Malleable
Unlike metals which can be hammered into sheets (malleability) or drawn into wires (ductility), nonmetals are generally brittle and lack malleability and ductility. When subjected to stress, they tend to shatter or crumble rather than deform. This is due to the strong covalent bonds holding their atoms together, which are less flexible than the metallic bonds in metals.
Examples: A piece of coal (carbon) will crumble when struck, demonstrating its brittleness. Sulfur, another nonmetal, also exhibits this characteristic.
3. Lower Density than Metals
Nonmetals typically have lower densities than metals. This means that a given volume of a nonmetal will weigh less than the same volume of a metal. This difference in density is related to the arrangement and bonding of atoms in each type of element.
Examples: Compare the density of iron (a metal) to the density of oxygen (a nonmetal). Oxygen gas is significantly less dense than iron. Even solid nonmetals like carbon (in the form of graphite) are less dense than many metals.
4. Dull Appearance (Lack of Luster)
Nonmetals generally lack the shiny, metallic luster characteristic of metals. They often appear dull or have a non-reflective surface. This is due to the way they interact with light; electrons in metals are free to interact with light, resulting in a reflective surface, while in nonmetals, the electrons are tightly bound, preventing this interaction.
Examples: Sulfur is a yellow, powdery substance that lacks the characteristic shine of metals. Carbon, in the form of charcoal, is a dull black substance.
5. Low Melting and Boiling Points
Most nonmetals have relatively low melting and boiling points compared to metals. This difference arises from the nature of the interatomic forces holding the atoms together. The weaker intermolecular forces in nonmetals require less energy to overcome, resulting in lower melting and boiling points.
Examples: Oxygen's boiling point is -183°C, while iron's boiling point is significantly higher at 2862°C. This vast difference highlights the contrasting properties of metals and nonmetals.
6. Formation of Covalent Bonds
Nonmetals tend to form covalent bonds with other nonmetals. In covalent bonding, atoms share electrons to achieve a stable electron configuration. This contrasts with metallic bonding in metals, where electrons are delocalized across a lattice of atoms. This covalent bonding influences many of the nonmetals' physical and chemical properties.
Examples: The molecule of water (H₂O) is formed by covalent bonds between oxygen and hydrogen (both nonmetals). Similarly, methane (CH₄) contains covalent bonds between carbon and hydrogen.
7. Variety of States at Room Temperature
Nonmetals can exist in different states at room temperature. Some are gases (like oxygen and nitrogen), others are solids (like carbon and sulfur), and one is a liquid (bromine). This diversity in physical states reflects the range of intermolecular forces present in different nonmetals.
Examples: Oxygen (gas), bromine (liquid), carbon (solid). This demonstrates the range of physical properties within the nonmetal group.
8. Nonmetals as Insulators (Electrical & Thermal)
This characteristic reiterates the point about conductivity. The structure and electron configuration of nonmetals make them excellent insulators. They are used extensively in electrical applications to prevent current flow and heat transfer.
Examples: Polymers (like plastics), ceramics, and rubber are used as insulators in wires and electronic components due to their non-conductive properties.
9. Gain Electrons to Form Negative Ions (Anions)
Nonmetals have a high electronegativity, meaning they have a strong tendency to attract electrons. When they react with metals, they gain electrons, forming negatively charged ions called anions. This is a key characteristic distinguishing them from metals which tend to lose electrons.
Examples: Chlorine readily gains an electron to become the chloride ion (Cl⁻). Oxygen gains two electrons to become the oxide ion (O²⁻).
10. Formation of Acids
Many nonmetal oxides react with water to form acids. These acids are often corrosive and contribute to the acidic nature of certain environmental phenomena, like acid rain.
Examples: Carbon dioxide (CO₂) reacts with water to form carbonic acid (H₂CO₃). Sulfur dioxide (SO₂) reacts with water to form sulfurous acid (H₂SO₃).
Differentiating Nonmetals from Metalloids
It's important to distinguish between nonmetals and metalloids. Metalloids, sometimes called semi-metals, possess properties intermediate between metals and nonmetals. They exhibit some metallic characteristics, such as conductivity, but also display some nonmetallic traits.
Examples of Metalloids: Silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), and tellurium (Te) all exhibit characteristics of both metals and nonmetals, making them metalloids and not strictly nonmetals. They are often semiconductors, possessing electrical conductivity that lies between metals and nonmetals.
Applications of Nonmetals
Nonmetals, despite their seemingly less glamorous properties compared to metals, are essential in numerous applications:
- Gases: Oxygen is vital for respiration, while nitrogen is crucial in fertilizer production. Noble gases like helium and neon find uses in lighting and other applications.
- Industrial Chemicals: Chlorine is used extensively in water purification, while sulfur is vital in the production of sulfuric acid, a cornerstone of many industrial processes.
- Construction: Sand (silicon dioxide) is a primary component of concrete and glass.
- Electronics: Silicon is the backbone of the semiconductor industry, crucial for the development of integrated circuits and microprocessors.
- Plastics and Polymers: Carbon is a key element in numerous plastics and polymers used in various applications.
Conclusion: Identifying Nonmetals
By understanding the key characteristics discussed – poor conductors, brittleness, low density, dull appearance, low melting points, covalent bonding, variety of states, insulating properties, anion formation, and acid formation – you can effectively identify nonmetals. Remember to distinguish them from metalloids, which have intermediate properties. Nonmetals, though perhaps less flashy than metals, are indispensable components in numerous everyday applications and are fundamental to our understanding of chemistry and the world around us. Their diverse properties contribute significantly to our modern technologies and understanding of natural processes.
Latest Posts
Latest Posts
-
What Is An Operator In Biology
Mar 17, 2025
-
How Many Symmetry Lines Does A Square Have
Mar 17, 2025
-
Do Viruses Belong To One Of The Domains Of Life
Mar 17, 2025
-
The C Shape Of The Tracheal Cartilages Is Important Because
Mar 17, 2025
-
Least Common Multiple Of 5 6 7
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is A Characteristic Of Nonmetals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
