Which Of The Following Elements Are Found In Glucose
Juapaving
Mar 30, 2025 · 7 min read
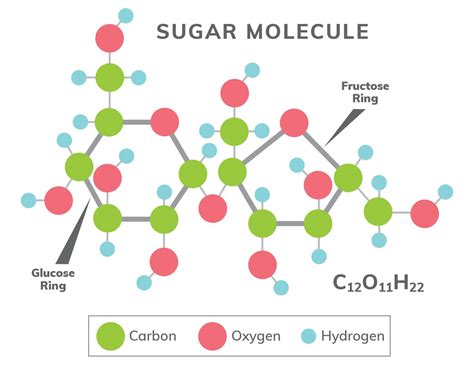
Table of Contents
Which Elements are Found in Glucose? A Deep Dive into the Molecular Composition of Sugar
Glucose, a simple sugar and the most abundant monosaccharide, is fundamental to life as we know it. Understanding its elemental composition is key to grasping its biological role and importance in various processes. This comprehensive guide delves into the precise elements found in glucose, exploring its molecular structure and the significance of each component. We'll also touch upon related concepts like isomers and the broader context of glucose in metabolism.
The Basic Building Blocks: Carbon, Hydrogen, and Oxygen
Glucose, like all carbohydrates, is primarily composed of three elements: carbon (C), hydrogen (H), and oxygen (O). These elements combine in a specific ratio to form the glucose molecule. The exact molecular formula for glucose is C₆H₁₂O₆. This formula clearly indicates that one molecule of glucose contains six carbon atoms, twelve hydrogen atoms, and six oxygen atoms.
The Role of Carbon: The Backbone of the Molecule
Carbon forms the backbone of the glucose molecule. Its unique ability to form four covalent bonds allows it to create a complex ring structure, essential for glucose's function. These carbon atoms are arranged in a six-membered ring, a structure commonly referred to as a pyranose ring. This ring structure is not planar but adopts a chair conformation, a three-dimensional arrangement that contributes to glucose's stability and reactivity. The specific arrangement of these carbons and their associated bonds dictates glucose's properties and interactions with other molecules.
The Importance of Hydrogen: Contributing to Reactivity and Stability
Hydrogen atoms are abundantly present in glucose. They are attached to the carbon atoms in the ring and also to oxygen atoms in the hydroxyl (-OH) groups. The presence of these hydroxyl groups is crucial. They contribute significantly to glucose's solubility in water, making it readily available for transport and utilization within living organisms. The hydrogen bonding between these hydroxyl groups and water molecules explains glucose's high solubility. The positioning of these hydrogens also influences the molecule's reactivity, determining how it interacts in metabolic pathways.
Oxygen's Crucial Role: Functional Groups and Reactivity
Oxygen atoms in glucose are primarily found within hydroxyl (-OH) groups and as part of the carbonyl group (C=O) in its open-chain form (although predominantly present in the ring structure). These oxygen atoms directly influence glucose's chemical reactivity and its ability to participate in various biochemical reactions. The hydroxyl groups are critical for the formation of glycosidic bonds, allowing glucose to link with other monosaccharides to form disaccharides (like sucrose) or polysaccharides (like starch and cellulose). The carbonyl group (in its open chain form), a reactive functional group, plays a key role in oxidation-reduction reactions that are integral parts of energy generation in cellular respiration.
Understanding the Molecular Structure: A Deeper Look at the Ring and Open-Chain Forms
Glucose exists in both a cyclic (ring) form and an open-chain form. While the ring form is predominantly present in solution, understanding both forms is crucial for comprehending its reactivity.
The Cyclic Form: The Predominant Structure
The cyclic form of glucose is a six-membered ring, also known as a pyranose ring. This ring is formed through the reaction between the aldehyde group (in the open-chain form) and a hydroxyl group on carbon 5. This intramolecular reaction creates a stable, cyclic structure. The ring contains five carbon atoms and one oxygen atom. The hydroxyl groups attached to the carbons in the ring can exist in different orientations, leading to different isomers (alpha and beta glucose). These isomers have slightly different properties and play distinct roles in various metabolic processes.
The Open-Chain Form: A Less Common but Significant Structure
The open-chain form of glucose is a linear structure with an aldehyde group (-CHO) at one end and several hydroxyl groups (-OH) along its carbon chain. This form is less prevalent in solution but is crucial for understanding glucose's reactivity. The aldehyde group is a highly reactive functional group, readily participating in redox reactions (reduction-oxidation) vital for energy production within cells. The equilibrium between the cyclic and open-chain forms ensures that a small proportion of glucose molecules are always in the reactive open-chain form, ready for participation in metabolic pathways.
Isomers of Glucose: Variations on a Theme
Glucose is an aldohexose, meaning it's an aldehyde sugar with six carbon atoms. However, the specific arrangement of the atoms and functional groups can lead to different isomers. These isomers, while having the same molecular formula (C₆H₁₂O₆), possess different structural arrangements and, therefore, different properties.
α-Glucose vs. β-Glucose: Key Differences
Two important isomers of glucose are α-glucose and β-glucose. They differ only in the orientation of the hydroxyl group attached to the first carbon atom (anomeric carbon) in the ring structure. In α-glucose, this hydroxyl group is below the plane of the ring, while in β-glucose, it is above the plane of the ring. This seemingly minor difference results in significant variations in their properties and roles in biological processes. For example, α-glucose is a key component of starch, whereas β-glucose is a building block of cellulose.
Other Glucose Isomers: A Wider Perspective
Beyond α-glucose and β-glucose, other isomers of glucose exist, though less commonly encountered in biological systems. These isomers can result from different arrangements of the atoms in the open-chain form or subtle variations in the ring structure. These variations often affect reactivity and interactions with other molecules.
Glucose's Role in Metabolism: A Central Player in Energy Production
The elemental composition of glucose directly influences its role in metabolism. The carbon atoms provide the framework for energy storage, the hydrogen atoms contribute to the energy released during oxidation, and the oxygen atoms participate in the redox reactions that generate ATP (adenosine triphosphate), the cell's primary energy currency.
Glycolysis: The First Step in Glucose Breakdown
Glycolysis is the initial stage of glucose catabolism (breakdown). This pathway occurs in the cytoplasm and involves a series of enzymatic reactions that break down glucose into two molecules of pyruvate. This process releases a small amount of ATP and NADH (a reducing agent), setting the stage for further energy production. The precise arrangement of carbon, hydrogen, and oxygen atoms in glucose is critical for the efficiency of these enzymatic reactions.
Cellular Respiration: Extracting Maximum Energy from Glucose
Cellular respiration is a more efficient process for extracting energy from glucose. It involves a series of reactions in the mitochondria that fully oxidize glucose, generating a significantly larger amount of ATP. This process requires the participation of oxygen, which accepts electrons at the end of the electron transport chain, forming water. The energy released during electron transfer is harnessed to generate a proton gradient that drives ATP synthesis. The specific arrangement of atoms in glucose is instrumental in facilitating this complex process.
Photosynthesis: Glucose Synthesis from Light Energy
Glucose isn't just broken down; it's also synthesized. Photosynthesis, the process by which plants and some other organisms convert light energy into chemical energy, produces glucose. This process uses carbon dioxide from the atmosphere, water, and light energy to synthesize glucose, storing solar energy in the chemical bonds of glucose. The precise composition of glucose is crucial for its effectiveness as an energy-rich molecule.
Conclusion: The Importance of Understanding Glucose's Elemental Composition
The elemental composition of glucose—carbon, hydrogen, and oxygen—is fundamental to understanding its structure, properties, and biological significance. The specific arrangement of these atoms dictates its reactivity, solubility, and its critical role in numerous metabolic processes. This knowledge is paramount in fields like biochemistry, medicine, and nutrition, contributing to advancements in various areas, including disease treatment, food science, and agricultural technology. The simplicity of its composition belies the immense complexity and importance of glucose in the living world.
Latest Posts
Latest Posts
-
Examples Of The Eight Parts Of Speech
Apr 01, 2025
-
Is Anything That Occupies Space And Has Mass
Apr 01, 2025
-
Mario Has To Take Medication For 180 Days
Apr 01, 2025
-
What Is On A Physical Map
Apr 01, 2025
-
P Block Elements In Periodic Table
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Elements Are Found In Glucose . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
