Is Anything That Occupies Space And Has Mass
Juapaving
Apr 01, 2025 · 7 min read
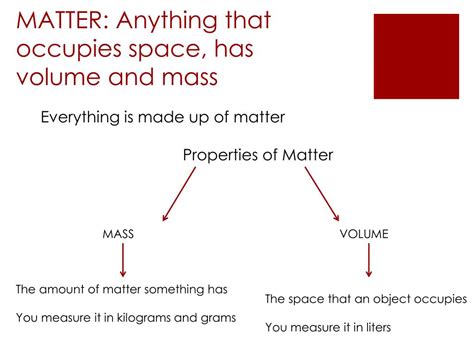
Table of Contents
Is Anything That Occupies Space and Has Mass: Exploring Matter and Its Properties
The simple statement, "Anything that occupies space and has mass," is a fundamental definition of matter. This seemingly straightforward concept underpins our understanding of the universe, from the smallest subatomic particles to the largest galaxies. But the nature of matter, its properties, and its various forms are far more complex and fascinating than this initial definition might suggest. This article delves deep into the concept of matter, exploring its various states, properties, and the scientific principles that govern its behavior.
What is Matter? A Deeper Dive
Matter, at its core, is anything that possesses both mass and volume (occupies space). Mass is a measure of the amount of matter an object contains, often associated with its inertia – its resistance to changes in motion. Volume, on the other hand, refers to the amount of three-dimensional space an object occupies. It’s crucial to understand that simply occupying space isn't enough; the object must also possess mass to be classified as matter. Light, for instance, occupies space and can exert pressure, but it lacks mass and therefore is not considered matter. Similarly, energy, while capable of transforming into matter (as described by Einstein's famous equation, E=mc²), is not matter itself in its fundamental state.
The Building Blocks of Matter: Atoms and Molecules
Matter is composed of fundamental building blocks known as atoms. Atoms are incredibly tiny particles consisting of a nucleus (containing protons and neutrons) surrounded by orbiting electrons. The number of protons determines the element to which an atom belongs (e.g., one proton for hydrogen, two for helium, etc.). Atoms of the same element can have different numbers of neutrons, resulting in isotopes.
Atoms rarely exist in isolation; they tend to bond together to form molecules. A molecule is a group of two or more atoms held together by chemical bonds. These bonds can be ionic (involving the transfer of electrons), covalent (involving the sharing of electrons), or metallic (involving a sea of delocalized electrons). The properties of a molecule are often significantly different from the properties of the individual atoms that compose it. Water (H₂O), for example, is a liquid at room temperature, whereas hydrogen and oxygen are gases.
States of Matter: From Solid to Plasma
Matter exists in various states, primarily defined by the arrangement and interaction of its constituent particles. The most common states are:
1. Solid
In a solid, atoms or molecules are tightly packed in a fixed, ordered arrangement. They possess strong intermolecular forces, resulting in a defined shape and volume. Solids are generally rigid and incompressible. Examples include ice, rock, and metal. Different types of solids exist, including crystalline solids (with a regular, repeating pattern) and amorphous solids (lacking a regular pattern).
2. Liquid
Liquids have weaker intermolecular forces than solids. Their atoms or molecules are less tightly packed and can move around relatively freely, allowing liquids to flow and take the shape of their container. However, liquids maintain a constant volume. Examples include water, oil, and mercury.
3. Gas
Gases have very weak intermolecular forces. Their atoms or molecules are widely dispersed and move rapidly and randomly. Gases have neither a definite shape nor a definite volume; they expand to fill their container. Examples include air, oxygen, and carbon dioxide.
4. Plasma
Plasma is often referred to as the fourth state of matter. It's a superheated gas in which atoms are ionized, meaning they have lost or gained electrons. This results in a mixture of positively charged ions and free-moving electrons. Plasma is found in stars, lightning, and fluorescent lights. It exhibits unique electrical and magnetic properties.
Properties of Matter: Physical and Chemical
Matter exhibits a wide range of properties, which can be broadly categorized as physical and chemical:
Physical Properties
These are characteristics that can be observed or measured without changing the substance's chemical composition. Examples include:
- Density: The mass per unit volume of a substance.
- Melting point: The temperature at which a solid changes to a liquid.
- Boiling point: The temperature at which a liquid changes to a gas.
- Solubility: The ability of a substance to dissolve in another substance.
- Color: The visual appearance of a substance.
- Conductivity: The ability of a substance to conduct heat or electricity.
- Hardness: Resistance to scratching or indentation.
- Malleability: The ability of a substance to be hammered into thin sheets.
- Ductility: The ability of a substance to be drawn into wires.
Chemical Properties
These describe how a substance reacts with other substances and its potential to undergo chemical changes. Examples include:
- Flammability: The ability of a substance to burn.
- Reactivity: How readily a substance reacts with other substances.
- Toxicity: The harmful effects of a substance on living organisms.
- Acidity/Basicity (pH): A measure of how acidic or basic a substance is.
- Oxidation: The ability of a substance to react with oxygen.
Changes in Matter: Physical and Chemical
Matter can undergo two main types of changes:
Physical Changes
These changes affect the physical properties of a substance but not its chemical composition. The substance remains the same; only its form or appearance alters. Examples include:
- Melting: Solid to liquid
- Freezing: Liquid to solid
- Boiling: Liquid to gas
- Condensation: Gas to liquid
- Sublimation: Solid to gas (e.g., dry ice)
- Deposition: Gas to solid (e.g., frost formation)
- Dissolving: A substance breaking down into smaller particles without changing its chemical nature.
Chemical Changes
These changes alter the chemical composition of a substance, resulting in the formation of new substances with different properties. These changes are often irreversible. Examples include:
- Burning (combustion): A substance reacts rapidly with oxygen, releasing heat and light.
- Rusting (oxidation): Iron reacts with oxygen and water to form iron oxide (rust).
- Digestion: The breakdown of food molecules into smaller molecules.
- Photosynthesis: Plants convert carbon dioxide and water into glucose and oxygen.
Conservation of Mass and Energy
A fundamental principle in the study of matter is the Law of Conservation of Mass, which states that mass cannot be created or destroyed in a chemical reaction. The total mass of the reactants equals the total mass of the products. However, this law needs refinement in the context of nuclear reactions, where a small amount of mass can be converted into energy (or vice versa), as described by Einstein's famous equation, E=mc². This leads to the Law of Conservation of Mass-Energy, which states that the total mass-energy of a closed system remains constant.
The Classification of Matter: Pure Substances and Mixtures
Matter can be further classified into pure substances and mixtures:
Pure Substances
These are substances with a uniform and definite composition throughout. They cannot be separated into simpler substances by physical means. Pure substances include:
- Elements: Substances composed of only one type of atom (e.g., oxygen, gold, iron).
- Compounds: Substances composed of two or more different types of atoms chemically bonded together (e.g., water, salt, sugar).
Mixtures
These are combinations of two or more substances that are not chemically bonded. They can be separated into their component substances by physical means. Mixtures can be either homogeneous (uniform composition throughout, e.g., saltwater) or heterogeneous (non-uniform composition, e.g., sand and water).
Beyond the Basics: Exploring Advanced Concepts
The study of matter extends far beyond the fundamental concepts discussed above. Advanced topics include:
- Quantum mechanics: The study of matter at the atomic and subatomic level, revealing the wave-particle duality of matter and the probabilistic nature of electron behavior.
- Nuclear physics: The study of the nucleus of the atom, including nuclear reactions, radioactivity, and nuclear energy.
- Particle physics: The study of the fundamental constituents of matter, including quarks, leptons, and bosons.
- Materials science: The study of the properties and applications of various materials, including metals, ceramics, polymers, and composites.
- Cosmochemistry: The study of the chemical composition of celestial bodies and the origin of elements in the universe.
Conclusion
The statement "Anything that occupies space and has mass" provides a foundational understanding of matter. However, the world of matter is vastly more intricate and fascinating than this simple definition suggests. From the fundamental building blocks of atoms to the diverse states and properties of matter, the subject offers endless opportunities for exploration and discovery. Understanding the nature of matter is crucial for advancements in various scientific fields, including chemistry, physics, materials science, and beyond. Further exploration into the various aspects of matter, utilizing the concepts and principles discussed in this article, will undoubtedly lead to a deeper and more comprehensive understanding of our physical world.
Latest Posts
Latest Posts
-
Period 1 Contains A Total Of Elements
Apr 02, 2025
-
Which Statements Are True Regarding Undefinable Terms In Geometry
Apr 02, 2025
-
What Is 3 Of 1 Million
Apr 02, 2025
-
In What Cell Organelle Does Photosynthesis Occur
Apr 02, 2025
-
Name 3 Kinds Of Hard Part Fossils
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Is Anything That Occupies Space And Has Mass . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
