Where Is The Mass Of The Atom Located
Juapaving
Mar 10, 2025 · 6 min read
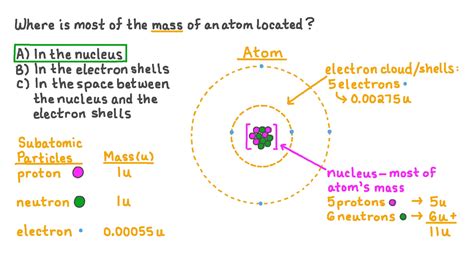
Table of Contents
Where Is the Mass of the Atom Located? Delving into the Nucleus
The atom, the fundamental building block of matter, is a fascinating realm of physics. Understanding its structure and the distribution of its mass is crucial to grasping the intricacies of chemistry, nuclear physics, and materials science. While the atom is often depicted as a miniature solar system with electrons orbiting a central nucleus, the reality is far more complex and nuanced. This article delves deep into the question: where is the mass of the atom located? We'll explore the subatomic particles, their properties, and how they contribute to the overall mass of an atom.
Unveiling the Subatomic World: Protons, Neutrons, and Electrons
The atom comprises three fundamental subatomic particles: protons, neutrons, and electrons. Each plays a critical role in determining the atom's properties, but their contributions to the overall mass differ significantly.
Protons: The Positively Charged Heavyweights
Protons reside within the atom's nucleus, a dense central region. They carry a positive electric charge, equal in magnitude but opposite in sign to the electron's charge. Crucially, protons contribute significantly to the atom's mass. Their mass is approximately 1.6726 × 10^-27 kg, often approximated as 1 atomic mass unit (amu). The number of protons in an atom's nucleus defines its atomic number, which uniquely identifies the element. For example, hydrogen has one proton (atomic number 1), helium has two (atomic number 2), and so on.
Neutrons: The Neutral Mass Contributors
Neutrons, also found within the nucleus, are electrically neutral particles. Their mass is slightly larger than that of a proton, approximately 1.6749 × 10^-27 kg, also roughly 1 amu. While they don't contribute to the atom's charge, neutrons play a vital role in determining the atom's mass and stability. The number of neutrons in an atom's nucleus can vary, leading to isotopes of the same element. Isotopes have the same number of protons but different numbers of neutrons, resulting in variations in their mass.
Electrons: The Lightweight Orbiters
Electrons are negatively charged particles that orbit the nucleus at considerable distances. Their mass is incredibly small, approximately 9.1094 × 10^-31 kg, which is nearly 2000 times less than the mass of a proton or neutron. Consequently, electrons contribute negligibly to the atom's overall mass. Their primary role lies in determining the atom's chemical properties and its interactions with other atoms. The number of electrons usually equals the number of protons in a neutral atom, ensuring a balanced charge.
The Nucleus: The Mass Hub of the Atom
From the above discussion, it becomes clear that the vast majority of an atom's mass is concentrated in its nucleus. The nucleus, a tiny, incredibly dense region at the atom's center, houses the protons and neutrons. The combined mass of these particles accounts for over 99.9% of the atom's total mass. The electrons, although crucial for chemical reactions and the overall electrical neutrality of the atom, contribute a negligible fraction to the total mass.
Isotopes and Mass Number: Variations in Atomic Mass
The concept of isotopes further reinforces the importance of the nucleus in determining atomic mass. Isotopes are atoms of the same element (same number of protons) that have different numbers of neutrons. This variation in neutron number leads to differences in their atomic mass. The mass number of an atom is the sum of its protons and neutrons. For example, carbon-12 has 6 protons and 6 neutrons (mass number 12), while carbon-14 has 6 protons and 8 neutrons (mass number 14). Both are isotopes of carbon, but their masses differ due to the varying number of neutrons. The relative abundance of different isotopes influences the average atomic mass reported on the periodic table.
Beyond Protons and Neutrons: Binding Energy and Mass Defect
The story doesn't end with just protons and neutrons. The mass of a nucleus is slightly less than the sum of the masses of its individual protons and neutrons. This difference is known as the mass defect. This "missing" mass is converted into binding energy, which holds the nucleus together. This phenomenon is a direct consequence of Einstein's famous equation, E=mc², where energy (E) and mass (m) are equivalent, related by the speed of light (c). The binding energy is a significant amount of energy, showcasing the powerful forces at play within the atomic nucleus. A greater binding energy signifies a more stable nucleus.
Atomic Mass Units (amu) and the Periodic Table
The atomic mass unit (amu) is a convenient unit for expressing the mass of atoms and molecules. One amu is defined as one-twelfth the mass of a carbon-12 atom. The periodic table lists the average atomic mass of each element, taking into account the relative abundance of its various isotopes. This average mass reflects the overall mass contribution from protons and neutrons, weighted by the natural abundance of each isotope.
Applications and Implications: Understanding Atomic Mass
Understanding where the mass of the atom is located has far-reaching implications across numerous scientific fields:
Nuclear Physics: Harnessing Nuclear Energy
The concentration of mass in the nucleus is central to nuclear physics. Nuclear reactions, such as nuclear fission and fusion, involve changes in the nucleus, releasing enormous amounts of energy. This energy is harnessed in nuclear power plants and nuclear weapons, highlighting the immense energy stored within the atom's core.
Chemistry: Isotope Applications and Chemical Reactions
Isotopes, with their varying neutron numbers and consequently varying masses, have important applications in various fields, including medicine and environmental science. Radioactive isotopes are used as tracers in biological and medical research. The differences in mass between isotopes can also subtly influence chemical reaction rates, although these effects are usually minor compared to the electronic effects.
Materials Science: Material Properties and Atomic Structure
The arrangement of atoms in a material, along with the atomic masses and the interactions between atoms, profoundly affects the material's overall properties. Understanding the atomic mass distribution helps in designing materials with specific characteristics, such as strength, conductivity, or reactivity.
Astrophysics: Stellar Nucleosynthesis and the Origin of Elements
The formation of elements in stars (stellar nucleosynthesis) involves nuclear reactions that create heavier elements from lighter ones. This process, deeply rooted in the mass-energy equivalence, is crucial to understanding the abundance of elements in the universe.
Medical Imaging: Radioisotopes in Diagnostics and Treatment
Radioactive isotopes are used extensively in medical imaging techniques like PET (positron emission tomography) and SPECT (single-photon emission computed tomography). These techniques rely on the decay of radioactive isotopes within the body to create images used for diagnosis and treatment monitoring.
Conclusion: A Deep Dive into Atomic Mass
In conclusion, the vast majority of an atom's mass is concentrated within its tiny nucleus. The protons and neutrons, with their relatively large masses compared to electrons, are the primary contributors. The number of protons defines the element, while the number of neutrons contributes to the isotope, influencing the atomic mass. The mass defect and binding energy further emphasize the intricate relationship between mass and energy within the nucleus. Understanding the distribution of mass within the atom is fundamental to various scientific fields, from nuclear physics and chemistry to materials science and astrophysics. The exploration of atomic structure continues to reveal deeper insights into the fundamental nature of matter and the universe itself.
Latest Posts
Latest Posts
-
Isotopes Of An Element Differ Due To The Number Of
Mar 10, 2025
-
The First Ten Elements Of The Periodic Table
Mar 10, 2025
-
Select The Components Of A Nucleotide
Mar 10, 2025
-
What Are The Common Factors Of 10 And 5
Mar 10, 2025
-
How Are Frequency And Period Related To Each Other
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about Where Is The Mass Of The Atom Located . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
