Isotopes Of An Element Differ Due To The Number Of
Juapaving
Mar 10, 2025 · 6 min read
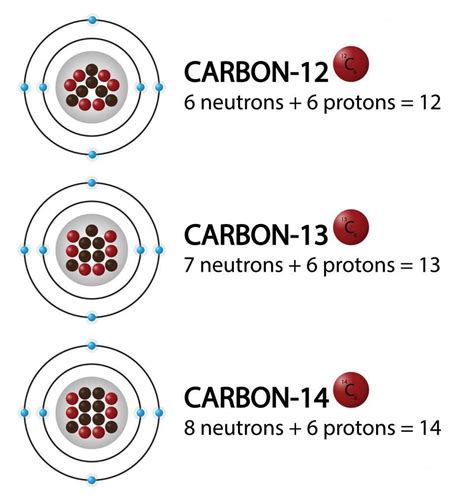
Table of Contents
Isotopes of an Element Differ Due to the Number of Neutrons
Isotopes are variations of a chemical element that possess the same number of protons but differ in the number of neutrons within their atomic nuclei. This seemingly subtle difference has profound implications for the element's properties, applications, and even its stability. Understanding isotopes is crucial in various scientific fields, from nuclear physics and chemistry to medicine and geology. This comprehensive article delves into the intricacies of isotopes, exploring their differences, classifications, applications, and significance.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before diving into the specifics of isotopes, it's essential to establish a firm understanding of atomic structure. An atom, the fundamental building block of matter, comprises three primary subatomic particles:
-
Protons: Positively charged particles residing within the atom's nucleus. The number of protons defines the element's atomic number and dictates its chemical identity. All atoms of a given element have the same number of protons. For example, all hydrogen atoms have one proton, all carbon atoms have six, and all oxygen atoms have eight.
-
Neutrons: Electrically neutral particles also found in the nucleus. Unlike protons, the number of neutrons in an atom can vary, even within the same element. This variation is the defining characteristic of isotopes.
-
Electrons: Negatively charged particles that orbit the nucleus in shells or energy levels. The number of electrons generally equals the number of protons in a neutral atom, ensuring a balanced charge. However, atoms can gain or lose electrons, forming ions with a net positive or negative charge.
What Makes Isotopes Different: The Neutron Count
The key difference between isotopes of the same element lies solely in the number of neutrons. This difference affects several crucial properties:
-
Mass Number: The mass number of an atom is the sum of its protons and neutrons. Since isotopes of an element have the same number of protons but different numbers of neutrons, they have different mass numbers. This is often represented as a superscript to the left of the element's symbol (e.g., ¹²C, ¹⁴C for carbon isotopes).
-
Nuclear Stability: The neutron-to-proton ratio significantly impacts an atom's nuclear stability. Some combinations of protons and neutrons create stable nuclei, while others result in unstable, radioactive isotopes. Radioactive isotopes decay over time, emitting particles or energy to achieve a more stable configuration. This decay process is fundamental to various applications, including radiometric dating and medical imaging.
-
Physical Properties: While the chemical properties of isotopes are largely identical due to the same number of electrons and protons, their physical properties, such as mass and density, can differ subtly. This difference becomes significant when dealing with large quantities of isotopes.
-
Chemical Properties: Despite the differing mass numbers, the chemical properties of isotopes of the same element remain largely unchanged. This is because chemical reactions are primarily determined by the electron configuration, which is not affected by the number of neutrons in the nucleus.
Types of Isotopes: Stable and Radioactive
Isotopes can be broadly classified into two categories:
-
Stable Isotopes: These isotopes possess a stable neutron-to-proton ratio and do not undergo radioactive decay. They are found naturally and constitute the majority of atoms in the environment. Examples include ¹²C (carbon-12), ¹⁶O (oxygen-16), and ¹⁴N (nitrogen-14).
-
Radioactive Isotopes (Radioisotopes): These isotopes have an unstable neutron-to-proton ratio and undergo radioactive decay to achieve stability. During decay, they emit various types of radiation, such as alpha particles, beta particles, or gamma rays. The rate of decay is characterized by the isotope's half-life, which is the time it takes for half of the radioactive atoms in a sample to decay. Examples include ¹⁴C (carbon-14), which is used in radiocarbon dating, and ¹³¹I (iodine-131), used in medical treatments.
Applications of Isotopes: A Diverse Range
The unique properties of isotopes, particularly radioisotopes, lead to their widespread applications in diverse fields:
1. Medicine:
- Diagnostics: Radioisotopes are employed in various medical imaging techniques, including PET (positron emission tomography) and SPECT (single-photon emission computed tomography) scans. These techniques use radioactively labeled compounds to visualize organs and tissues, aiding in the diagnosis of diseases like cancer and heart conditions.
- Treatment: Radioisotopes are also used in radiation therapy to target and destroy cancerous cells. Specific radioisotopes, chosen for their decay characteristics and targeting capabilities, are administered to patients to selectively irradiate the tumor.
- Tracers: Radioisotopes act as tracers to track biological processes. By incorporating a radioisotope into a molecule, researchers can monitor its movement and metabolism within the body.
2. Industry:
- Gauging and Measurement: Radioisotopes are used in gauging thickness, density, and level of materials in various industrial processes. This is particularly useful in manufacturing and quality control.
- Sterilization: Gamma radiation from radioisotopes is used to sterilize medical equipment, food products, and other materials by killing bacteria and other microorganisms.
3. Environmental Science:
- Pollution Monitoring: Radioisotopes are utilized as tracers to study the movement of pollutants in the environment, such as water contamination and air pollution.
- Dating Techniques: Radiocarbon dating, based on the decay of ¹⁴C, is a crucial technique used to determine the age of organic materials, providing insights into archaeology, paleontology, and climate science.
4. Geology and Archaeology:
- Radiometric Dating: Various radioisotopes, with varying half-lives, are employed to date geological formations and artifacts. This helps to reconstruct the Earth's history and understand past civilizations. Potassium-argon dating, uranium-lead dating, and rubidium-strontium dating are some examples.
5. Research:
- Nuclear Physics: Studying the properties and behavior of various isotopes is crucial in furthering our understanding of nuclear physics, including nuclear reactions and radioactive decay processes.
- Chemical Analysis: Isotope ratios can be used in various analytical techniques to provide insights into chemical processes and pathways.
Isotopic Abundance and Average Atomic Mass
The relative abundance of different isotopes of an element in nature varies. This variation is usually expressed as a percentage. For example, carbon has two stable isotopes, ¹²C and ¹³C, with abundances of approximately 98.9% and 1.1%, respectively. The average atomic mass of an element is a weighted average of the masses of its isotopes, considering their relative abundances. This average atomic mass is the value usually reported on the periodic table.
Conclusion: The Significance of Isotopes
The concept of isotopes is fundamental to understanding the structure and behavior of matter. The subtle differences in neutron number lead to significant variations in properties, making isotopes invaluable tools in numerous scientific and technological applications. From medical diagnostics and treatment to geological dating and industrial processes, isotopes play a vital role in shaping our understanding of the world and advancing technological progress. Further research into the properties and applications of isotopes promises to unlock even more potential benefits in the future. The continuing study of isotopes underscores their importance as versatile probes for exploring the fundamental aspects of matter and its interactions within diverse environments and systems.
Latest Posts
Latest Posts
-
List Three Substances Typically Found In Glomerular Filtrate
Mar 10, 2025
-
What Is A Row Called In The Periodic Table
Mar 10, 2025
-
Is Sound Potential Or Kinetic Energy
Mar 10, 2025
-
Is 34 A Prime Number Or A Composite Number
Mar 10, 2025
-
Which Of The Following Lists Disadvantages Of Nuclear Power
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about Isotopes Of An Element Differ Due To The Number Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
