Where In The Periodic Table Are Metals Found
Juapaving
Mar 17, 2025 · 5 min read
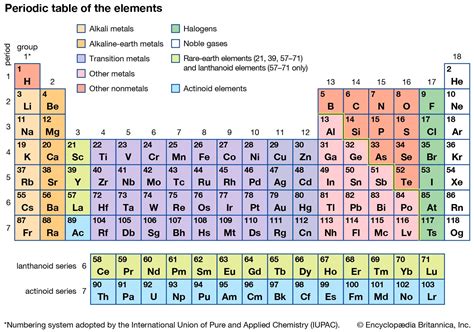
Table of Contents
Where in the Periodic Table are Metals Found? A Comprehensive Guide
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. One of the most fundamental classifications within this system is the division between metals and non-metals. Understanding where metals reside on the periodic table is crucial to comprehending their characteristics and applications. This article delves deep into the location of metals within the periodic table, exploring their properties, exceptions, and the fascinating nuances of this classification.
The Broad Sweep: Metals Dominate the Left
The most straightforward answer to the question "Where in the periodic table are metals found?" is: primarily on the left and in the center. A vast majority of elements categorized as metals occupy the left two-thirds of the periodic table. This isn't a strict line, however; the boundary between metals and non-metals is a gradual transition zone, often referred to as the metalloids or semimetals.
The Staircase: A Visual Guide to Metal Location
A helpful visual cue for identifying metals lies in the staircase-like line running diagonally from boron (B) to astatine (At). Elements generally to the left of this line are metals. Those to the right are non-metals, with the elements directly on the line exhibiting properties of both, hence their designation as metalloids. This line is not a perfect delineator, and there are some exceptions, but it provides a good starting point for understanding metal location.
Exploring Metal Families: Properties and Positions
Metals are not a homogeneous group; they exhibit a wide range of properties, often related to their specific location within the periodic table. Different groups or families of metals share similar characteristics due to their similar electron configurations.
Alkali Metals (Group 1): Highly Reactive Leftmost Residents
Located in the first column (Group 1), alkali metals are exceptionally reactive due to their single valence electron. Their reactivity increases as you move down the group. These soft, silvery-white metals are never found in nature in their pure form, always bonded with other elements. Examples include lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). Their extreme reactivity necessitates careful handling and storage.
Alkaline Earth Metals (Group 2): Reactive, but Less Than Alkali Metals
Next to the alkali metals, in Group 2, reside the alkaline earth metals. These metals are also highly reactive, although less so than their alkali metal counterparts, because they have two valence electrons. Like alkali metals, they are never found uncombined in nature. Examples include beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). These metals find various applications, from construction (magnesium and calcium) to lighting (strontium).
Transition Metals (Groups 3-12): A Diverse Central Bloc
The central block of the periodic table, encompassing groups 3 to 12, contains the transition metals. This is the largest group of metals, exhibiting a greater diversity of properties than other metal families. Their valence electrons reside in more than one shell, contributing to their varied oxidation states and complex ion formations. Transition metals are known for their strength, hardness, and ability to form colored compounds, often used in pigments and catalysts. Iron (Fe), copper (Cu), gold (Au), and platinum (Pt) are just a few examples from this rich and important group.
Lanthanides and Actinides: The Inner Transition Metals
Below the main body of the periodic table, are the lanthanides and actinides. These elements, often referred to as the inner transition metals, are placed separately for formatting reasons, but they belong to periods 6 and 7, respectively. They share similar chemical properties and are characterized by their partially filled f orbitals. Many lanthanides find use in alloys and magnets, while the actinides are known for their radioactivity.
Post-Transition Metals: A Bridge to Metalloids
Found to the right of the transition metals, the post-transition metals demonstrate properties bridging the gap between the classic metallic characteristics and the non-metallic properties of metalloids. They are relatively soft and have lower melting points than most transition metals. Examples include aluminum (Al), tin (Sn), and lead (Pb). Aluminum is renowned for its lightness and corrosion resistance, while lead is now less widely used due to toxicity concerns.
Metalloids: The Borderline Cases
The metalloids, also known as semimetals, occupy a critical zone along the staircase line, exhibiting properties of both metals and non-metals. Their conductivity, for instance, can be influenced by factors like temperature and pressure. Silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), and tellurium (Te) are key examples. These elements play crucial roles in semiconductors and electronics due to their unique conductivity characteristics.
Exceptions and Nuances: Not Every Element Fits Neatly
While the broad categorization is helpful, there are exceptions and subtle nuances to consider. The properties of elements can vary depending on the specific context, such as temperature and pressure, thus blurring the lines between metal and non-metal classifications.
Beyond the Periodic Table: Practical Applications
Understanding the location of metals in the periodic table is far from a purely academic exercise. This knowledge is fundamental to:
- Material Science: Predicting the properties of new alloys and compounds.
- Chemistry: Understanding chemical reactions and bonding.
- Engineering: Selecting appropriate materials for different applications based on their strength, conductivity, and other properties.
- Electronics: Designing semiconductors and other electronic components.
- Medicine: Developing new drugs and therapies (e.g., using platinum-based drugs in cancer treatment).
Conclusion: A Dynamic and Ever-Evolving Understanding
The location of metals in the periodic table offers a powerful framework for understanding their properties and applications. While the broad classification provides a helpful overview, the nuances and exceptions highlight the complexity and dynamism of the periodic table itself. Continued research and exploration constantly refine our understanding of the properties and behavior of elements, leading to further advances in numerous fields. Remember the staircase, but be aware of the exceptions! This comprehensive understanding is crucial for advancements in material science, chemistry, engineering, and countless other scientific endeavors. The periodic table serves as a vital roadmap, guiding our explorations into the fascinating world of elements and their diverse applications.
Latest Posts
Latest Posts
-
How Many Symmetry Lines Does A Square Have
Mar 17, 2025
-
Do Viruses Belong To One Of The Domains Of Life
Mar 17, 2025
-
The C Shape Of The Tracheal Cartilages Is Important Because
Mar 17, 2025
-
Least Common Multiple Of 5 6 7
Mar 17, 2025
-
How Do You Find The Inverse Of A Relation
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Where In The Periodic Table Are Metals Found . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
