What's The Difference Between Electron Geometry And Molecular Geometry
Juapaving
Apr 01, 2025 · 5 min read
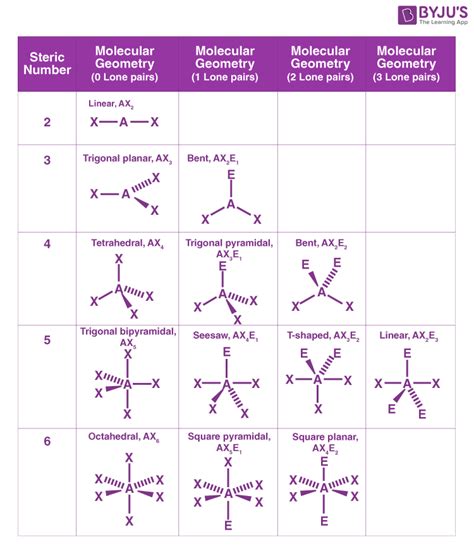
Table of Contents
What's the Difference Between Electron Geometry and Molecular Geometry?
Understanding the three-dimensional arrangement of atoms within a molecule is crucial in chemistry. This arrangement dictates a molecule's properties, including its reactivity, polarity, and physical state. Two closely related concepts, electron geometry and molecular geometry, are often confused, yet understanding their differences is key to predicting molecular behavior. This article delves deep into the nuances of each, highlighting their similarities and crucial distinctions.
Understanding Electron Geometry: The Complete Picture
Electron geometry describes the arrangement of all electron pairs surrounding the central atom in a molecule, including both bonding pairs (electrons involved in covalent bonds) and lone pairs (non-bonding electrons). It focuses on the spatial distribution of these electron pairs to minimize repulsion between them. This principle, known as VSEPR theory (Valence Shell Electron Pair Repulsion), forms the foundation for predicting electron geometry. The electron pairs arrange themselves as far apart as possible to achieve the lowest energy state.
Key Factors Determining Electron Geometry
Several key factors influence the electron geometry of a molecule:
- Number of electron pairs: The total number of electron pairs (bonding and lone pairs) around the central atom dictates the basic electron geometry.
- Repulsive forces: Lone pairs exert stronger repulsive forces than bonding pairs due to their closer proximity to the central atom. This results in slight distortions in the overall geometry.
- Central atom: The nature of the central atom, its size, and its electronegativity can subtly influence the geometry, although the number of electron pairs remains the primary determinant.
Common Electron Geometries
Here are some of the most common electron geometries predicted by VSEPR theory:
- Linear: Two electron pairs arranged 180° apart. Example: BeCl₂
- Trigonal planar: Three electron pairs arranged 120° apart in a flat, triangular arrangement. Example: BF₃
- Tetrahedral: Four electron pairs arranged 109.5° apart in a three-dimensional tetrahedron. Example: CH₄
- Trigonal bipyramidal: Five electron pairs arranged in a trigonal bipyramid. This geometry has two distinct bond angles: 90° and 120°. Example: PCl₅
- Octahedral: Six electron pairs arranged 90° apart in an octahedral shape. Example: SF₆
Understanding Molecular Geometry: Focusing on Atoms
Molecular geometry, also known as molecular shape, describes the spatial arrangement of only the atoms in a molecule. Unlike electron geometry, it only considers the positions of the atoms bonded to the central atom, ignoring the lone pairs. While lone pairs influence the molecular geometry by affecting bond angles, they are not explicitly included in the description of the shape.
The Impact of Lone Pairs on Molecular Geometry
Lone pairs exert significant influence on molecular geometry. Because they occupy more space than bonding pairs, they compress the bond angles between the atoms. This leads to deviations from the idealized electron geometries.
Common Molecular Geometries
Let's revisit some of the electron geometries and see how the presence of lone pairs affects the corresponding molecular geometries:
- Linear (Electron Geometry): If all electron pairs are bonding pairs, the molecular geometry is also linear. Example: BeCl₂ (linear electron geometry and linear molecular geometry)
- Trigonal Planar (Electron Geometry):
- Trigonal Planar (Molecular Geometry): All three electron pairs are bonding pairs. Example: BF₃
- Bent (Molecular Geometry): Two bonding pairs and one lone pair. The lone pair repels the bonding pairs, causing a less than 120° bond angle. Example: SO₂
- Tetrahedral (Electron Geometry):
- Tetrahedral (Molecular Geometry): All four electron pairs are bonding pairs. Example: CH₄
- Trigonal Pyramidal (Molecular Geometry): Three bonding pairs and one lone pair. The lone pair compresses the bond angles below 109.5°. Example: NH₃
- Bent (Molecular Geometry): Two bonding pairs and two lone pairs. The lone pairs significantly compress the bond angles. Example: H₂O
- Trigonal Bipyramidal (Electron Geometry): The molecular geometry in this case can be several different shapes, depending on the positions of lone pairs (axial vs equatorial). Examples: PCl₅ (trigonal bipyramidal molecular geometry), SF₄ (see-saw), ClF₃ (T-shaped).
- Octahedral (Electron Geometry): Similar to the trigonal bipyramidal geometry, various molecular shapes are possible depending on the number and position of lone pairs. Examples: SF₆ (octahedral molecular geometry), BrF₅ (square pyramidal), XeF₄ (square planar).
Key Differences Summarized
The following table summarizes the key differences between electron geometry and molecular geometry:
| Feature | Electron Geometry | Molecular Geometry |
|---|---|---|
| Focus | Arrangement of all electron pairs (bonding & lone) | Arrangement of atoms only |
| Consideration | Includes lone pairs | Excludes lone pairs |
| Repulsion | Considers repulsion between all electron pairs | Considers repulsion between bonding pairs and lone pairs indirectly |
| Shape Prediction | Predicts the overall electron distribution | Predicts the shape of the molecule based on atomic positions |
| VSEPR Theory | Directly applied | Indirectly applied; lone pairs affect the shape |
Why is this distinction important?
The distinction between electron geometry and molecular geometry is crucial for several reasons:
- Predicting polarity: Molecular geometry determines the overall polarity of a molecule. Even if individual bonds are polar, the symmetrical arrangement of atoms in a molecule (e.g., CO₂) can lead to a nonpolar molecule.
- Understanding reactivity: The shape of a molecule directly impacts its reactivity. Specific functional groups and the accessibility of atoms influence how a molecule interacts with other molecules.
- Explaining physical properties: Molecular geometry influences physical properties like boiling point, melting point, and solubility.
- Spectroscopy: Molecular geometry is essential for interpreting spectroscopic data, such as infrared and Raman spectroscopy.
Advanced Concepts and Applications
Understanding electron and molecular geometries extends beyond simple molecules. It plays a crucial role in:
- Organic chemistry: Predicting the shape of complex organic molecules helps in understanding their reactivity and biological function.
- Inorganic chemistry: Electron and molecular geometries are essential for understanding the structure and bonding in coordination complexes and other inorganic compounds.
- Materials science: The geometry of molecules significantly influences the properties of materials, enabling the design of materials with specific characteristics.
- Computational chemistry: Sophisticated computational methods are used to predict and refine the electron and molecular geometries of molecules.
Conclusion
The distinction between electron geometry and molecular geometry is a cornerstone of understanding molecular structure and behavior. While seemingly subtle, the differences have profound implications for predicting molecular properties and reactivity. By mastering these concepts and applying VSEPR theory, chemists gain a powerful tool for understanding the intricate world of molecules and their interactions. Remember, electron geometry provides the complete picture of electron distribution, while molecular geometry focuses on the arrangement of atoms, highlighting the crucial influence of lone pairs on the final molecular shape. This nuanced understanding is vital for various fields within chemistry and beyond.
Latest Posts
Latest Posts
-
The Most Abundant Gas In The Earths Atmosphere Is
Apr 02, 2025
-
Is Rubbing Alcohol And Denatured Alcohol The Same
Apr 02, 2025
-
Is 17 A Prime Number Or A Composite Number
Apr 02, 2025
-
Is Burning A Candle A Chemical Or Physical Change
Apr 02, 2025
-
What Is The State Of Matter Of Fire
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What's The Difference Between Electron Geometry And Molecular Geometry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
