What Type Of Mixture Is Air
Juapaving
Mar 05, 2025 · 5 min read
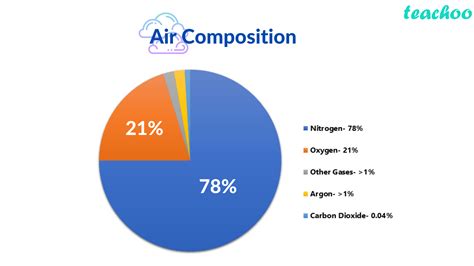
Table of Contents
What Type of Mixture is Air? A Deep Dive into the Composition and Properties of Earth's Atmosphere
Air. We breathe it, we live in it, yet how much do we truly understand about this ubiquitous mixture? While seemingly simple, air's composition and behavior are surprisingly complex. This article explores the nature of air as a mixture, delving into its constituent components, physical properties, and the implications of its heterogeneous nature.
Defining Mixtures: A Quick Review
Before diving into the specifics of air, let's establish a clear understanding of what constitutes a mixture. In chemistry, a mixture is a substance comprising two or more components not chemically bonded. These components retain their individual chemical properties and can be separated using physical methods like filtration, distillation, or evaporation. Mixtures contrast with compounds, where components are chemically bound and their individual properties are lost. Mixtures are further categorized as homogeneous or heterogeneous.
Homogeneous vs. Heterogeneous Mixtures
- Homogeneous mixtures: These have a uniform composition throughout. A solution, like saltwater, is a prime example. No matter where you sample the solution, the ratio of salt to water remains constant.
- Heterogeneous mixtures: These have a non-uniform composition. The components are visibly distinct, and their proportions vary depending on the location of the sample. A salad, for instance, is a heterogeneous mixture; the lettuce, tomatoes, and dressing are readily distinguishable.
Air: A Heterogeneous Mixture
Air, the lifeblood of our planet, is undeniably a mixture. It's not a single substance but a collection of various gases, liquids (in the form of water vapor), and even solid particles (dust, pollen, etc.). Importantly, air is a heterogeneous mixture. The distribution of its components isn't uniform across the entire atmosphere.
The Major Components of Air
While the exact composition of air can vary depending on location, altitude, and weather conditions, the major components remain consistent. Dry air, meaning air with no water vapor, is primarily composed of:
- Nitrogen (N₂): Approximately 78% of dry air is nitrogen. This relatively inert gas plays a crucial role in maintaining the Earth's temperature balance.
- Oxygen (O₂): Making up roughly 21% of dry air, oxygen is essential for respiration in most living organisms. It's a highly reactive gas, crucial for combustion and numerous other chemical processes.
- Argon (Ar): This inert noble gas constitutes about 0.93% of dry air.
- Carbon Dioxide (CO₂): While a relatively small component (around 0.04%), carbon dioxide plays a significant role in the Earth's climate through the greenhouse effect. Its concentration is increasing due to human activities.
- Other Trace Gases: The remaining percentage comprises trace gases such as neon, helium, methane, krypton, hydrogen, and xenon. Although present in minute amounts, these gases can have significant environmental impacts.
The Variable Component: Water Vapor
Water vapor, the gaseous phase of water, is a highly variable component of air. Its concentration depends heavily on temperature and humidity. Warm, humid air can contain significantly more water vapor than cold, dry air. The presence of water vapor influences air density, temperature, and weather patterns.
Particulates in Air: Aerosols
Air also contains various solid and liquid particles, collectively known as aerosols. These include:
- Dust: Particles of soil, sand, and other materials lifted into the atmosphere by wind.
- Pollen: Tiny grains released by flowering plants, often causing allergies.
- Sea Salt: Particles of salt carried by wind from ocean spray.
- Soot and Smoke: Particles resulting from incomplete combustion of fossil fuels and other materials.
- Volcanic Ash: Particles ejected during volcanic eruptions.
These aerosols can affect air quality, visibility, and even cloud formation. Their presence further contributes to the heterogeneous nature of air.
The Implications of Air's Heterogeneous Nature
The fact that air is a heterogeneous mixture has several crucial implications:
Variable Properties
Because air's composition isn't uniform, its physical properties like density and refractive index aren't constant. These properties vary depending on location and atmospheric conditions. This variability is essential for understanding weather patterns and atmospheric dynamics.
Air Pollution and Its Impact
The presence of pollutants and aerosols in air directly impacts air quality. High concentrations of pollutants can pose serious health risks and contribute to environmental problems such as acid rain and smog. Monitoring and managing the concentration of these components is crucial for maintaining a healthy environment.
Altitude and Compositional Changes
The composition of air changes significantly with altitude. The lower atmosphere, the troposphere, contains the majority of the water vapor and aerosols. As altitude increases, the concentration of oxygen and other gases decreases, while the proportion of lighter gases, such as helium, may increase. The stratosphere, for example, contains a higher concentration of ozone (O3), which absorbs harmful ultraviolet radiation.
Regional Variations
The composition of air can also vary significantly depending on geographical location. Industrial areas may have higher concentrations of pollutants, while coastal regions may have higher concentrations of sea salt aerosols. These regional variations underscore the heterogeneous nature of the air we breathe.
Separating the Components of Air
Given that air is a mixture, its components can be separated using various physical methods. These methods exploit the differing physical properties of the constituent gases:
- Liquefaction: Cooling air to extremely low temperatures (-196°C for nitrogen, for example) causes its components to condense into liquids. These liquid components can then be separated through fractional distillation, taking advantage of their different boiling points.
- Membrane Separation: Specialized membranes can selectively filter certain gases from air, allowing for the separation of specific components.
- Absorption: Certain materials can selectively absorb particular gases from air.
Conclusion: Air – A Dynamic and Complex Mixture
In conclusion, air is unequivocally a heterogeneous mixture. Its complex composition, fluctuating due to factors like altitude, location, and weather, significantly influences Earth's climate, weather patterns, and overall environmental health. Understanding the heterogeneous nature of air is fundamental to comprehending atmospheric science, environmental protection, and the very air we breathe. The dynamic interplay of its various components highlights its complexity and underscores the importance of continued research and monitoring of this vital resource. Further research continues to unravel the subtle interactions between these components and their impact on our planet. This ongoing investigation continues to refine our understanding of this seemingly simple, yet profoundly complex, mixture. The study of air's composition and behavior remains a crucial aspect of numerous scientific disciplines, from meteorology and climatology to chemistry and environmental science.
Latest Posts
Latest Posts
-
Alternate Forms Of The Same Gene Are Called
Mar 05, 2025
-
Radius Of Convergence And Interval Of Convergence Calculator
Mar 05, 2025
-
60 Inches Is How Many Feet
Mar 05, 2025
-
Is Rusting Iron A Chemical Change
Mar 05, 2025
-
What Is The Lcm Of 5 And 6
Mar 05, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Mixture Is Air . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
