What Type Of Bond Is Kcl
Juapaving
Mar 19, 2025 · 6 min read
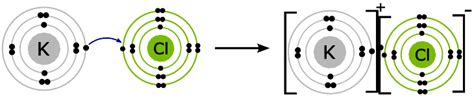
Table of Contents
What Type of Bond is KCl? Exploring the Ionic Bond in Potassium Chloride
Potassium chloride (KCl), a common salt found in nature and widely used in various applications, presents a fascinating example of chemical bonding. Understanding the type of bond in KCl is crucial to grasping its properties and behavior. This article delves deep into the nature of the bond in KCl, exploring its ionic character, formation, properties, and applications. We will also examine how the ionic bond influences its solubility, conductivity, and other key characteristics.
The Ionic Bond: A Strong Electrostatic Attraction
The fundamental type of bond present in KCl is an ionic bond. Unlike covalent bonds where atoms share electrons, ionic bonds involve the complete transfer of electrons from one atom to another. This transfer creates charged particles called ions: positively charged cations and negatively charged anions.
In KCl, potassium (K) is a metal with a single electron in its outermost shell. Chlorine (Cl), a nonmetal, has seven electrons in its outermost shell. Potassium readily loses its single valence electron to achieve a stable, filled electron shell (like noble gas Argon). Chlorine, on the other hand, readily gains this electron to complete its own outer shell, also achieving a stable noble gas configuration (like Argon).
This electron transfer results in the formation of a potassium cation (K⁺) and a chloride anion (Cl⁻). The electrostatic attraction between these oppositely charged ions is what constitutes the ionic bond. The strong Coulombic force between the positively charged potassium ion and the negatively charged chloride ion holds them together in a crystal lattice structure.
Understanding Electronegativity Differences
The significant difference in electronegativity between potassium and chlorine is the driving force behind the formation of this ionic bond. Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. Potassium has a low electronegativity, meaning it readily loses electrons. Chlorine, conversely, has a high electronegativity, strongly attracting electrons. This large difference in electronegativity facilitates the complete transfer of electrons, leading to the formation of ions and the ionic bond.
The Crystal Lattice Structure of KCl
The KCl crystal structure isn't a random arrangement of ions. Instead, it forms a highly organized, three-dimensional cubic lattice. This structure is characterized by a repeating pattern of K⁺ and Cl⁻ ions arranged in a way that maximizes electrostatic attraction and minimizes repulsion. Each K⁺ ion is surrounded by six Cl⁻ ions, and each Cl⁻ ion is surrounded by six K⁺ ions. This arrangement ensures that the positive and negative charges are balanced throughout the lattice, leading to a stable and energetically favorable structure.
The strong electrostatic forces within the crystal lattice contribute significantly to KCl's physical properties, such as its high melting and boiling points. A large amount of energy is required to overcome these strong attractive forces and break apart the crystal lattice.
Properties of KCl Influenced by its Ionic Bond
The ionic bonding in KCl dictates many of its observable properties. Let's examine some key examples:
High Melting and Boiling Points:
The strong electrostatic attractions between the ions in the crystal lattice necessitate a significant amount of energy to overcome them. This results in KCl's relatively high melting point (770°C) and boiling point (1420°C) compared to substances with weaker intermolecular forces.
Solubility in Water:
KCl is highly soluble in water. Water is a polar solvent, meaning it has a positive and negative end due to the polar O-H bonds. The positive end of water molecules is attracted to the Cl⁻ ions, and the negative end is attracted to the K⁺ ions. This interaction, known as hydration, weakens the electrostatic forces holding the KCl lattice together, allowing the ions to dissolve and become surrounded by water molecules.
Electrical Conductivity:
Solid KCl is a poor conductor of electricity because the ions are held rigidly in the crystal lattice and are not free to move. However, when KCl is dissolved in water or melted, the ions become mobile, allowing the solution or molten KCl to conduct electricity effectively. The free movement of the charged ions enables the transport of electric charge.
Brittle Nature:
Ionic compounds like KCl are generally brittle. When subjected to stress, the ions in the lattice can shift, bringing ions of the same charge into close proximity. This leads to strong repulsive forces that overcome the attractive forces, causing the crystal to fracture along cleavage planes.
Applications of Potassium Chloride
KCl's unique properties make it invaluable in several applications:
-
Agriculture: KCl is a crucial source of potassium, an essential macronutrient for plant growth. Potassium plays a critical role in various plant processes, including enzyme activation, protein synthesis, and stomatal regulation. It's used as a fertilizer to improve crop yields and quality.
-
Medicine: KCl is used in intravenous solutions to treat potassium deficiencies (hypokalemia). It's also employed in some lethal injection protocols, although its use in this context is ethically controversial and subject to ongoing debate.
-
Food Industry: KCl serves as a salt substitute in some food products, especially for individuals needing to reduce their sodium intake for health reasons. It provides a salty flavor, although slightly different from NaCl.
-
Industry: KCl finds application in various industrial processes, including the production of potassium hydroxide (KOH), other potassium salts, and certain types of fertilizers. It's also utilized in drilling fluids and as a component in some types of welding fluxes.
Distinguishing KCl's Ionic Bond from Other Bond Types
It's important to distinguish the ionic bond in KCl from other bond types:
-
Covalent Bonds: In covalent bonds, atoms share electrons rather than transferring them completely. This results in molecules with relatively lower melting and boiling points and different electrical conductivity properties compared to ionic compounds. Examples include water (H₂O) and methane (CH₄).
-
Metallic Bonds: Metallic bonds occur between metal atoms, involving a "sea" of delocalized electrons that are shared among many atoms. This leads to high electrical and thermal conductivity and malleability, unlike the brittle nature of ionic compounds. Examples include copper (Cu) and iron (Fe).
-
Hydrogen Bonds: Hydrogen bonds are relatively weak intermolecular forces that occur between molecules containing hydrogen atoms bonded to highly electronegative atoms (like oxygen or nitrogen). They significantly influence the properties of substances like water, but are not the primary bonding force in KCl.
Conclusion: The Defining Role of the Ionic Bond in KCl
The ionic bond is the defining characteristic of potassium chloride. The complete transfer of electrons from potassium to chlorine results in the formation of a stable crystal lattice held together by strong electrostatic forces. This ionic bonding profoundly impacts KCl's properties, including its high melting and boiling points, solubility in water, electrical conductivity (in solution or molten state), and brittle nature. These properties, in turn, dictate its wide range of applications in agriculture, medicine, food processing, and various industrial processes. Understanding the ionic bond in KCl is essential for appreciating its significance in diverse scientific and technological fields. Further research into the intricacies of ionic bonding continues to refine our understanding of its influence on material behavior and pave the way for innovative applications.
Latest Posts
Latest Posts
-
What Is The Sum Of Interior Angles Of A Rectangle
Mar 19, 2025
-
Energy Can Be From One Form To Another
Mar 19, 2025
-
50 As A Product Of Prime Factors
Mar 19, 2025
-
Which Of The Following Is A Property Of An Acid
Mar 19, 2025
-
Why Is A Plant Classified As An Autotroph
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Bond Is Kcl . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
