What Type Of Bond Holds The Nitrogen Bases Together
Juapaving
Mar 16, 2025 · 5 min read
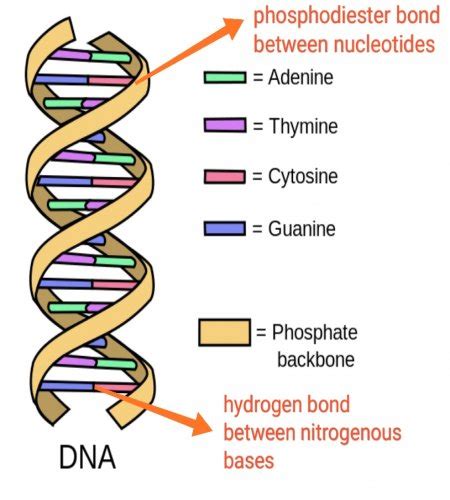
Table of Contents
What Type of Bond Holds the Nitrogen Bases Together? A Deep Dive into Hydrogen Bonding in DNA and RNA
The elegance and simplicity of the double helix structure of DNA, the blueprint of life, hinges on a seemingly simple yet crucial interaction: hydrogen bonding between nitrogenous bases. This seemingly weak bond is the powerhouse behind the stability of the genetic code, driving replication, transcription, and ultimately, the expression of life itself. Understanding the specifics of this bonding is crucial to comprehending the fundamental mechanisms of molecular biology. This article delves deep into the nature of these bonds, exploring their strength, specificity, and the implications of their unique properties.
The Players: Nitrogenous Bases and Their Structure
Before delving into the bonds themselves, let's briefly review the key players: the nitrogenous bases. These are the fundamental building blocks of nucleic acids (DNA and RNA), and their arrangement dictates the genetic code. There are five primary nitrogenous bases: adenine (A), guanine (G), cytosine (C), thymine (T), and uracil (U). A, G are purines (double-ring structures), while C, T, and U are pyrimidines (single-ring structures).
Purines: Adenine and Guanine
Adenine (A) and guanine (G), being purines, possess a larger, more complex structure. Their double-ring structure allows for multiple sites capable of forming hydrogen bonds. The specific arrangement of these sites is critical to their pairing specificity.
Pyrimidines: Cytosine, Thymine, and Uracil
Cytosine (C), thymine (T), and uracil (U), the pyrimidines, are structurally simpler, featuring a single-ring structure. While structurally simpler, their hydrogen bonding potential is equally crucial. Thymine is found exclusively in DNA, while uracil replaces thymine in RNA. This subtle difference has significant implications for the overall structure and function of these nucleic acids.
Hydrogen Bonding: The Glue of the Double Helix
The nitrogenous bases pair up specifically in DNA and RNA through hydrogen bonds. These are relatively weak bonds compared to covalent bonds (such as those within the sugar-phosphate backbone), but their collective strength, coupled with the stacking interactions of the bases, provides the stability necessary for DNA's double helix structure. The specificity of these bonds ensures the correct pairing of bases during DNA replication and transcription.
Adenine-Thymine (A-T) Pairing
Adenine (A) always pairs with thymine (T) in DNA through two hydrogen bonds. The specific arrangement of hydrogen bond donor (N-H) and acceptor (C=O) groups on A and T ensures this precise pairing. The precise geometry allows for optimal hydrogen bond formation, contributing to the stability of the double helix.
Guanine-Cytosine (G-C) Pairing
Guanine (G) pairs with cytosine (C) through three hydrogen bonds. This higher number of hydrogen bonds makes the G-C base pair slightly stronger than the A-T base pair. This difference in bond strength influences the melting temperature of DNA, the temperature at which the double helix separates into single strands. Regions of DNA rich in G-C pairs have higher melting temperatures.
Adenine-Uracil (A-U) Pairing
In RNA, where thymine is replaced by uracil, adenine (A) pairs with uracil (U) through two hydrogen bonds. The structural similarity between T and U allows for this analogous pairing, ensuring the correct base pairing during RNA transcription and other RNA-related processes.
The Specificity and Importance of Hydrogen Bonding
The specificity of hydrogen bonding between nitrogenous bases is paramount for several reasons:
-
Accurate Replication: During DNA replication, the hydrogen bonds between the base pairs must break to allow the two DNA strands to separate. The precise pairing ensures that each strand serves as an accurate template for the synthesis of a new complementary strand, maintaining the integrity of the genetic information.
-
Accurate Transcription: Transcription, the process of synthesizing RNA from a DNA template, relies on the specific base pairing between DNA and RNA. This process ensures the accurate transfer of genetic information from DNA to RNA.
-
Protein Synthesis: The sequence of nitrogenous bases in mRNA determines the sequence of amino acids in proteins. The accurate base pairing during transcription is essential for the accurate synthesis of proteins, which are vital for cellular structure and function.
-
DNA Structure and Stability: The collective strength of hydrogen bonds, combined with base stacking interactions (hydrophobic interactions between the planar aromatic rings of the bases), contributes to the overall stability of the DNA double helix. This stability is crucial for protecting the genetic information from damage.
Beyond Hydrogen Bonds: Other Contributing Factors to DNA Stability
While hydrogen bonding plays the primary role in base pairing, other interactions also contribute to the overall stability of the DNA double helix:
-
Base Stacking: The hydrophobic nature of the nitrogenous bases leads to favorable stacking interactions between adjacent bases. These interactions contribute significantly to the overall stability of the DNA double helix.
-
Hydrophobic Effects: The bases are relatively hydrophobic, leading to their clustering within the double helix, away from the surrounding water molecules. This hydrophobic effect further stabilizes the structure.
-
Electrostatic Interactions: Ionic interactions between the negatively charged phosphate groups and positively charged ions in the surrounding solution also contribute to DNA stability.
The Impact of Mutations: When Base Pairing Goes Wrong
Errors in base pairing, whether through spontaneous mutations or the action of mutagens, can have significant consequences. These errors can lead to mismatches during replication, causing insertions, deletions, or substitutions in the DNA sequence. Such changes can have devastating effects on the organism, resulting in genetic diseases or even cell death. Cells have evolved sophisticated mechanisms to repair these errors, minimizing the impact of such mutations.
Conclusion: A Delicate Balance of Forces
The type of bond holding nitrogen bases together, the seemingly weak yet crucial hydrogen bond, is a testament to the elegance of biological systems. The specificity and collective strength of these bonds, in conjunction with other stabilizing interactions, provide the foundation for the stable and accurate transmission of genetic information. Understanding this fundamental interaction is critical to comprehending the complexity and beauty of life itself. Further research into the nuances of hydrogen bonding and its role in DNA and RNA continues to unveil the remarkable intricacies of molecular biology. The ongoing study promises to shed further light on the mechanisms behind genetic stability, replication errors, and the development of therapeutic interventions targeting these critical processes. The intricate dance between hydrogen bonds, base stacking, and other interactions ultimately dictates the stability and function of the genetic material, shaping the very essence of life as we know it. The field remains dynamic and continues to expand our understanding of this fundamental process.
Latest Posts
Latest Posts
-
Write 50 As A Product Of Prime Factors
Mar 16, 2025
-
What Is Found In Both Dna And Rna
Mar 16, 2025
-
Rotational Symmetry Letters Of The Alphabet
Mar 16, 2025
-
What Is The Outermost Layer Of The Sun Called
Mar 16, 2025
-
What Are The Common Factors Of 25
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Bond Holds The Nitrogen Bases Together . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
